Bonding Exam Review
Link to Slides
https://
docs.
google.
com/presentation/d/1xXqtEk_dhhofafI3S1rZN2vKapBPgZ_igvXlVDCn0FE/edit?usp=sharing
Test Topics
Know the difference between covalent, ionic, and metallic bonds.
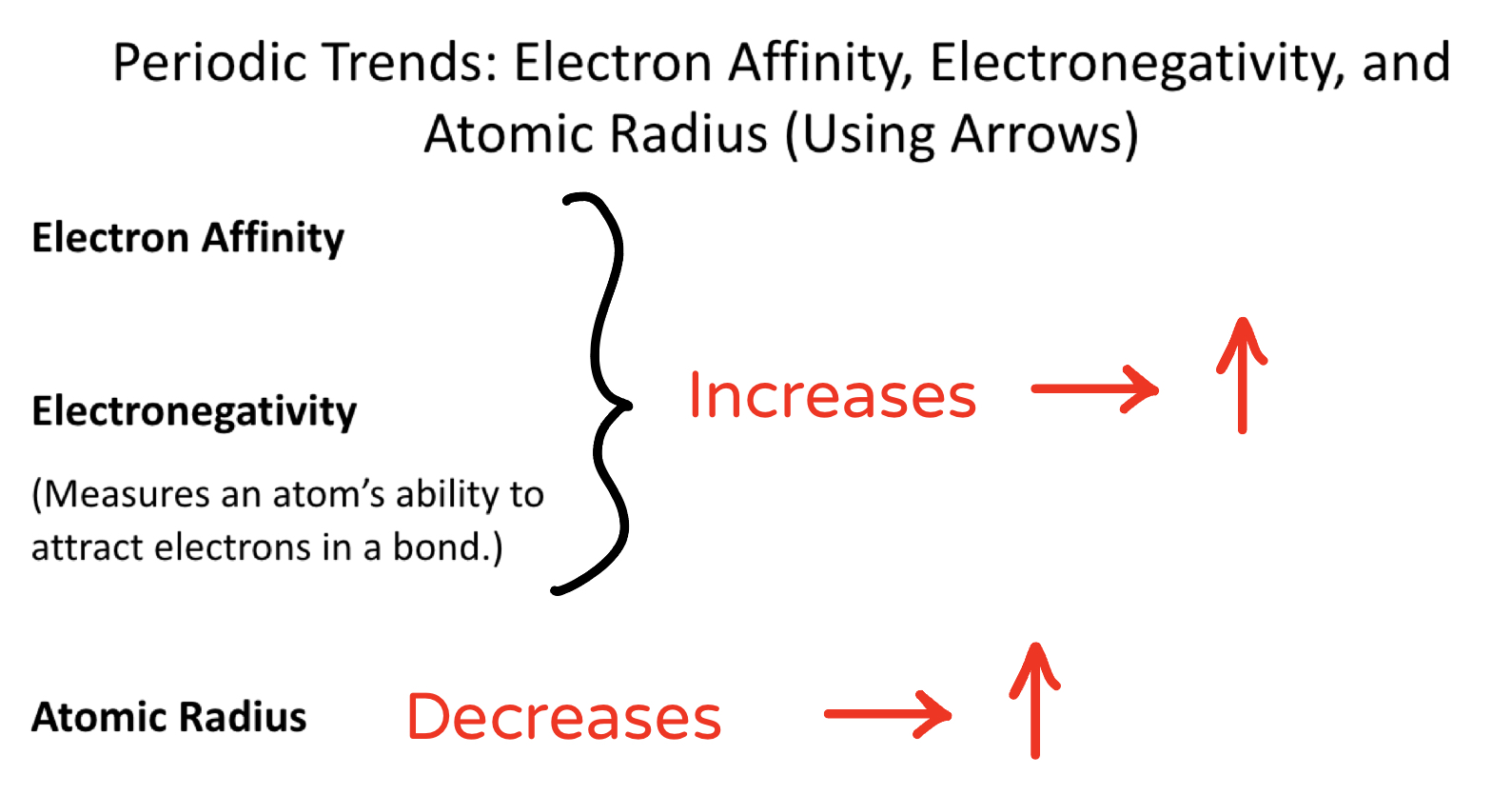
Know the definition of electronegativity
Know the trends for electron affinity, electronegativity, and atomic radius on the periodic table.
Be able to determine which out of 2 atoms is more electronegative.
Know that Fluorine is the most electronegative element and why.
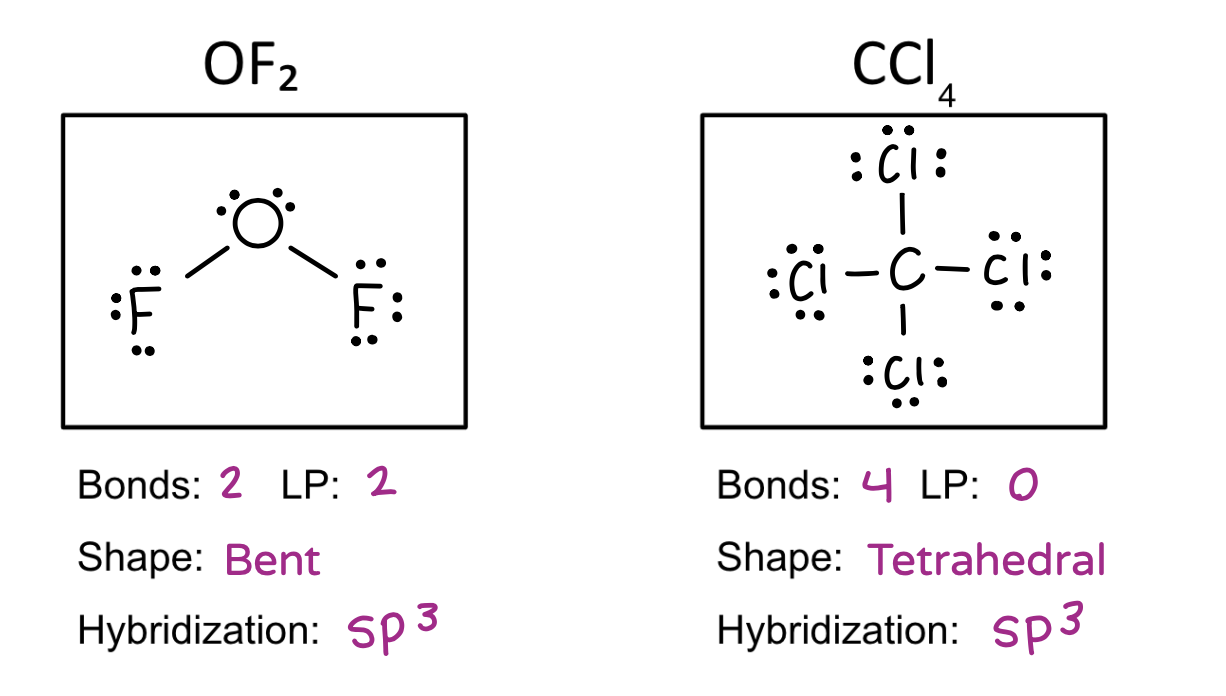
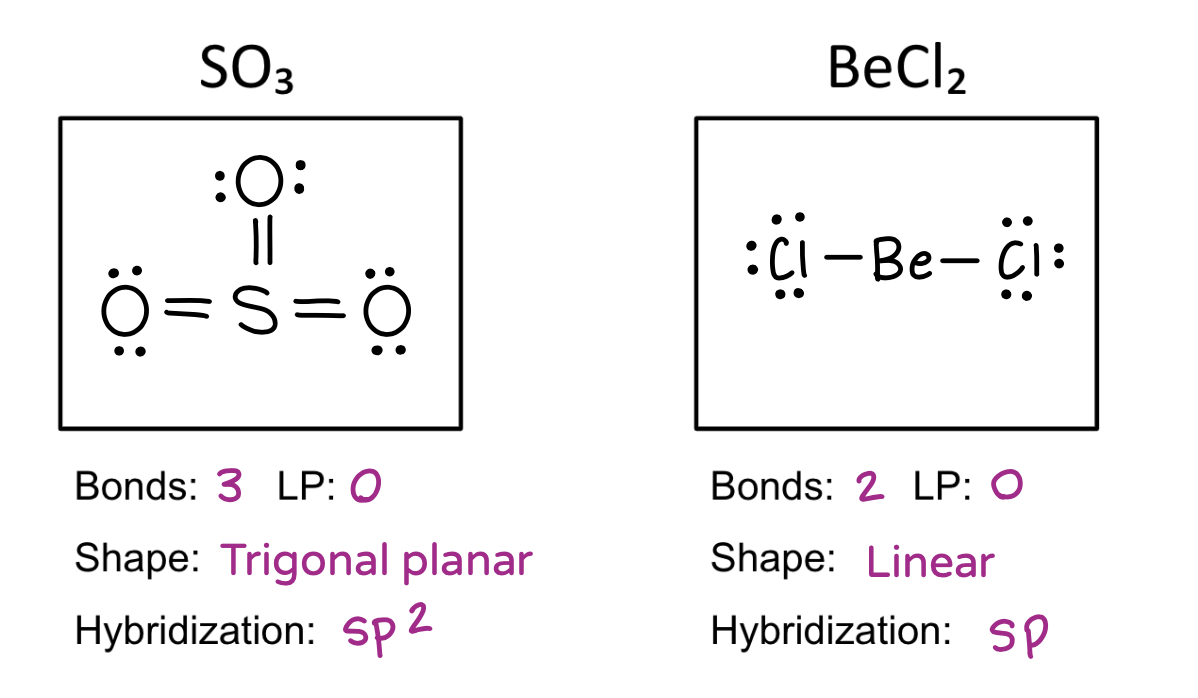
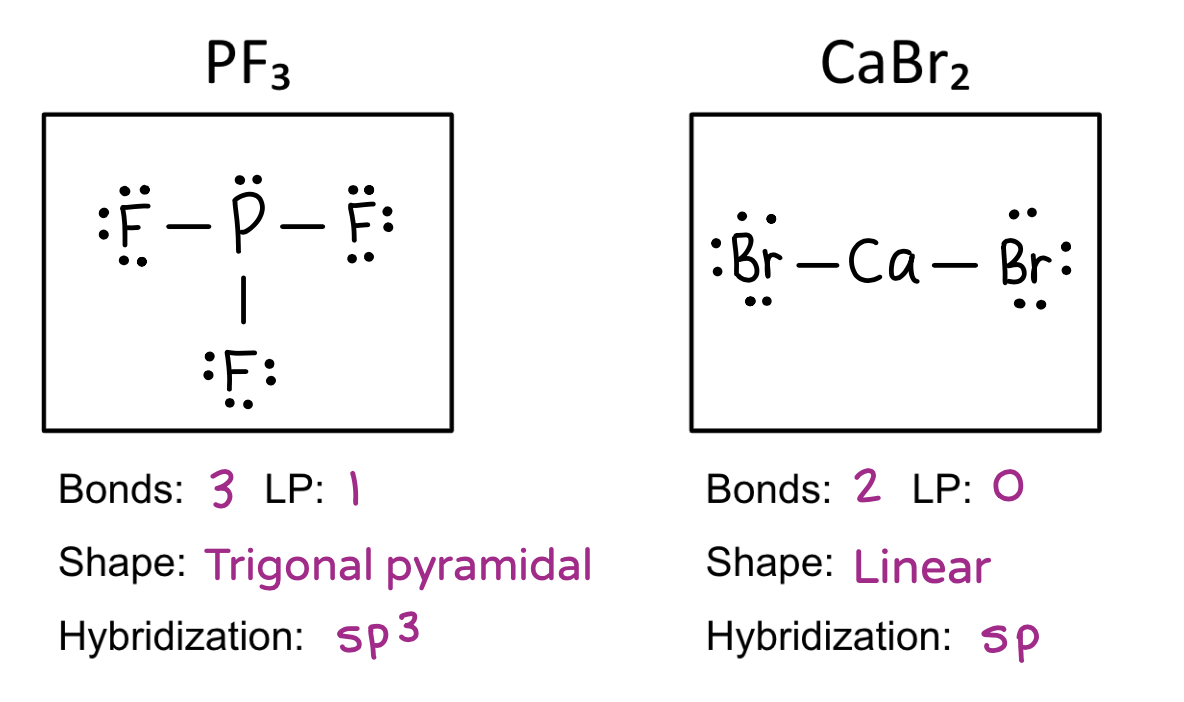
Be able to draw molecular structures, determine the molecular shape, calculate the electronegativity difference, name the bond type, and identify the hybridization.
What needs to be memorized
Molecular geometry
Linear
Bent
Trigonal planar
Trigonal pyramidal
Tetrahedral
Scale for different types of bonds
Less that 0.3 = Non-polar
0.31-1.8 = Polar
Greater than 1.8 = Ionic
Hybridization table
Atoms + Lone Pairs on central atom
4 = sp3
3 = sp2
2 = sp