Atomic Theory and Chemical Composition (DONE)
0.0(0)
0.0(0)
Card Sorting
1/117
Earn XP
Description and Tags
118 Questions
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
118 Terms
1
New cards
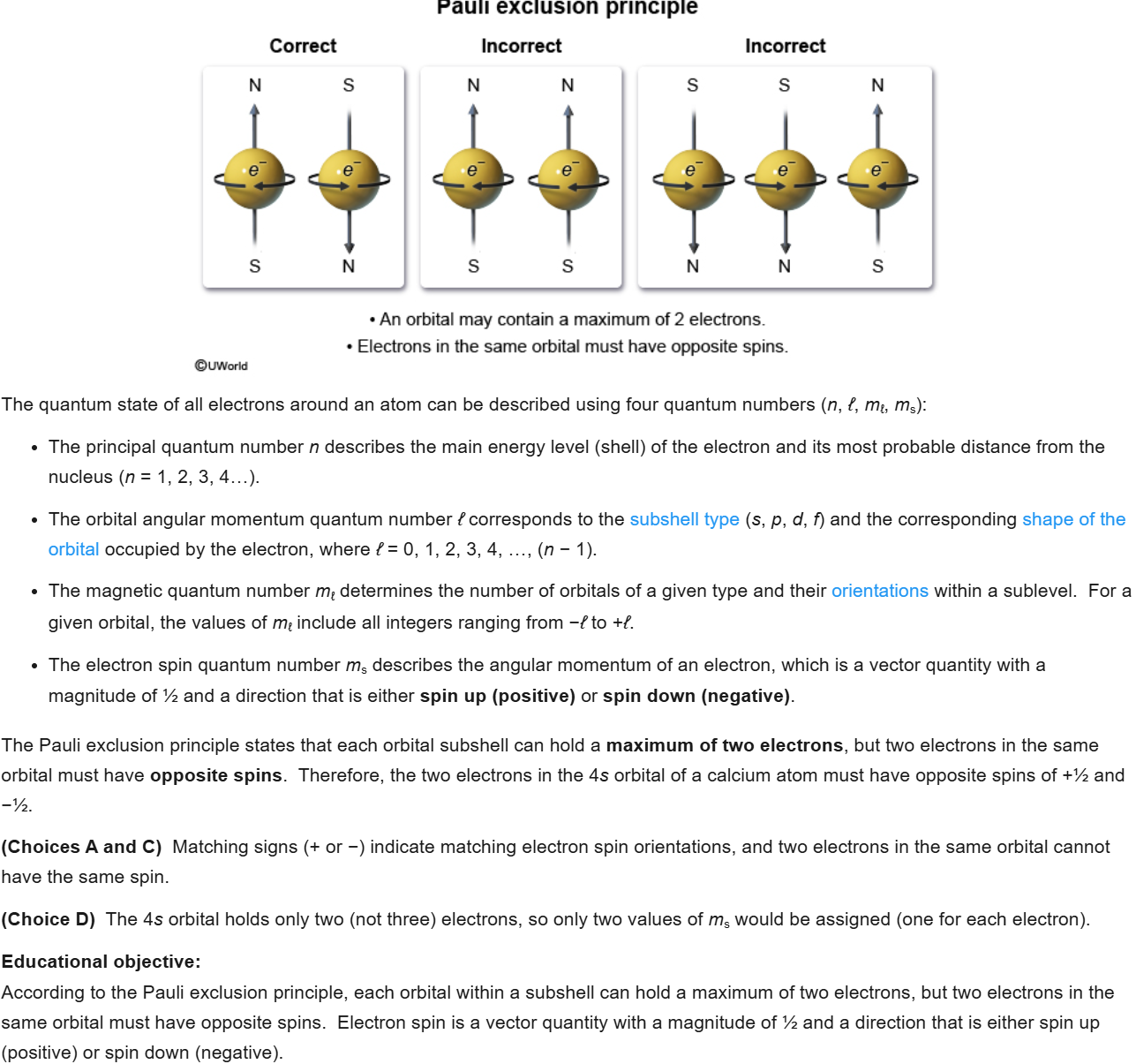
D

2
New cards

C- I and II
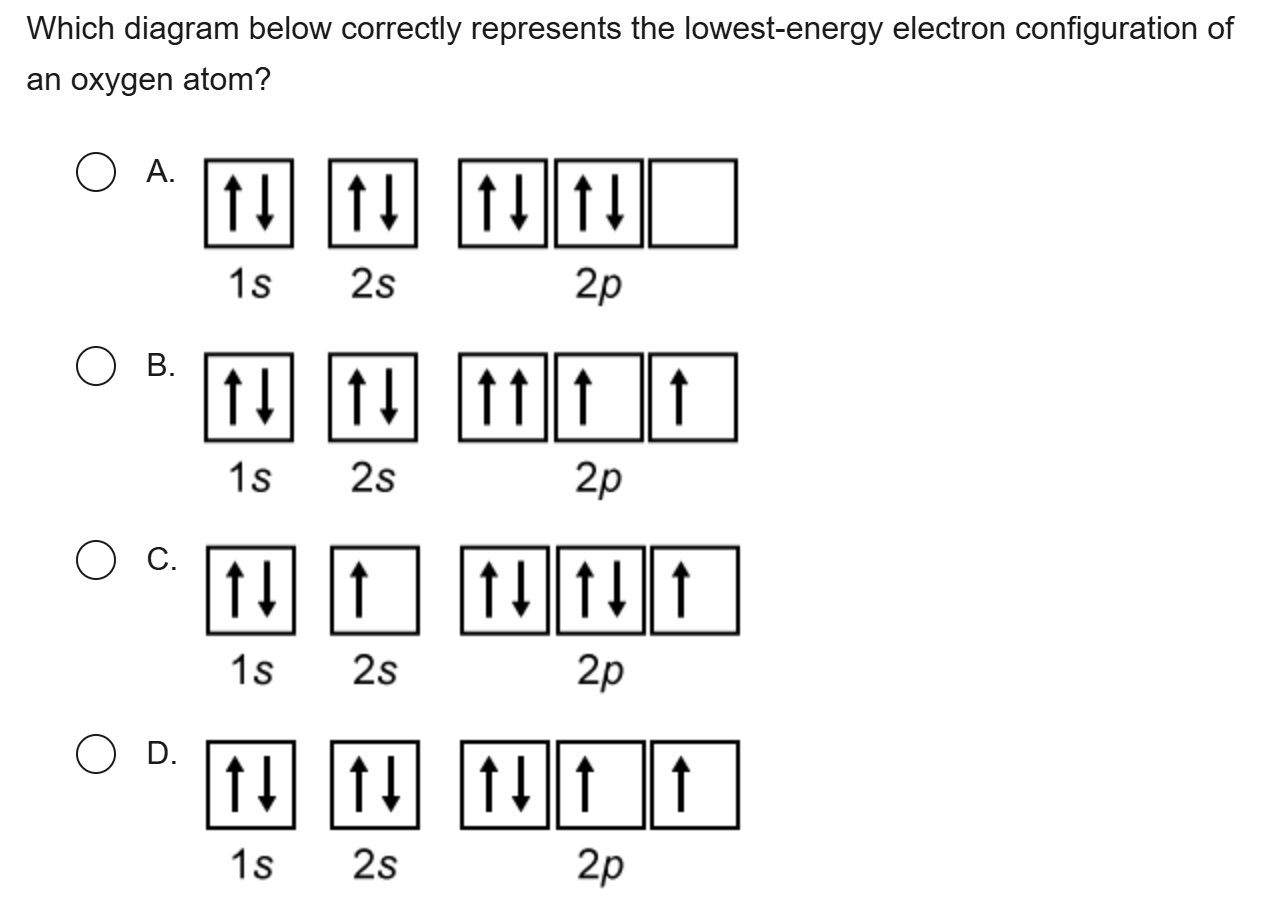
3
New cards
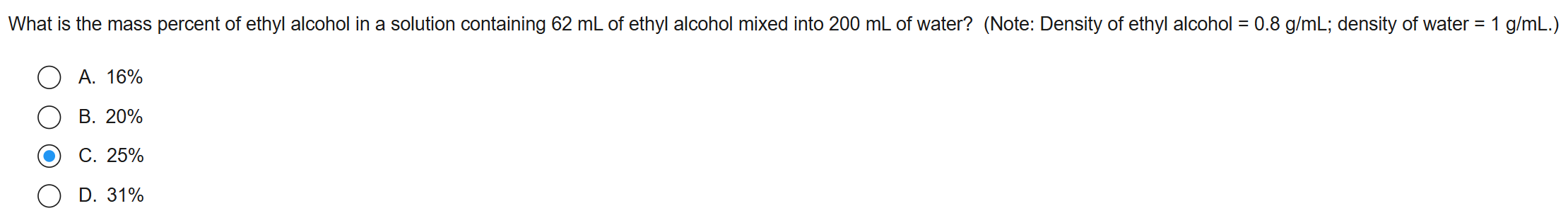
B
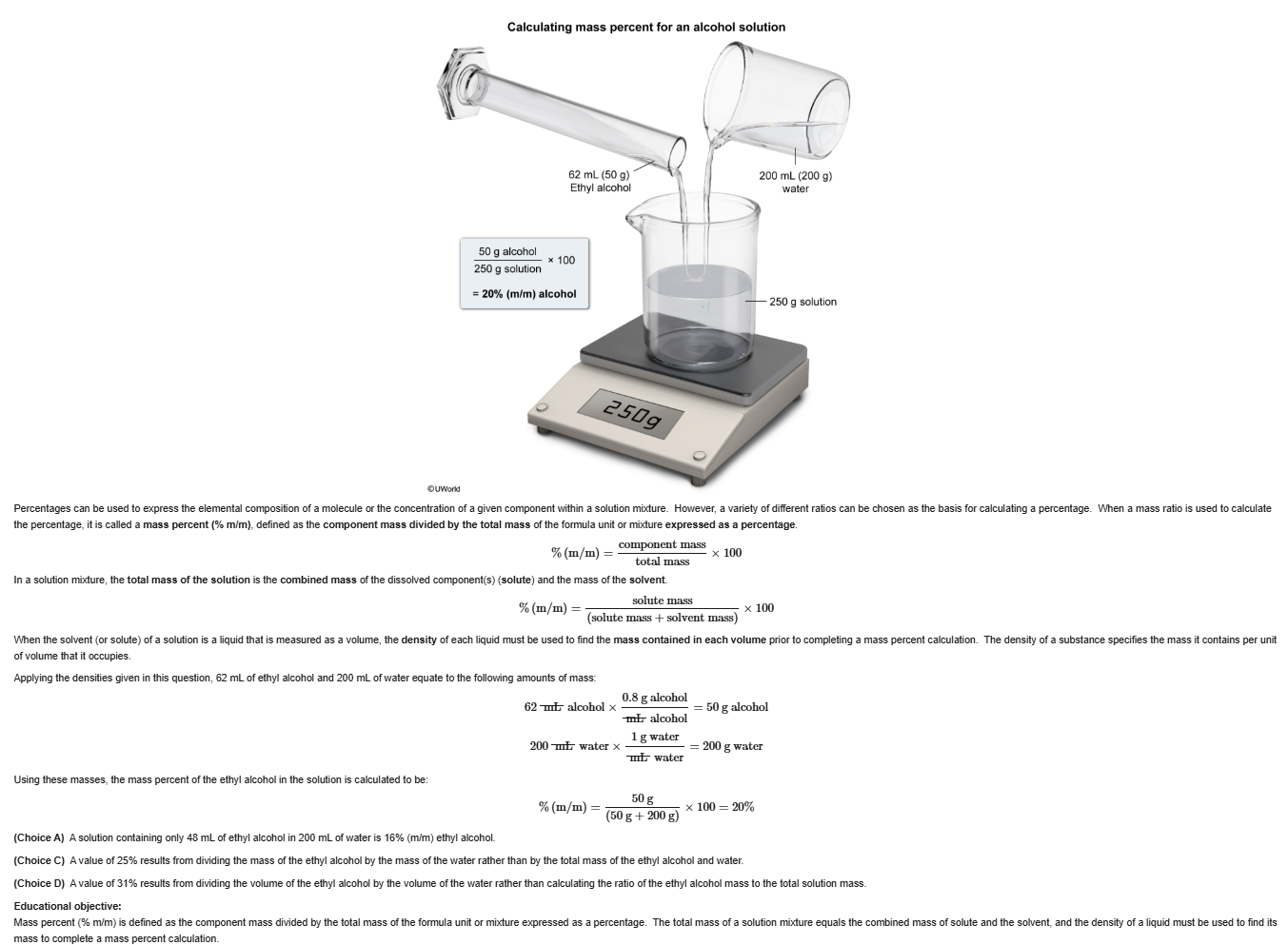
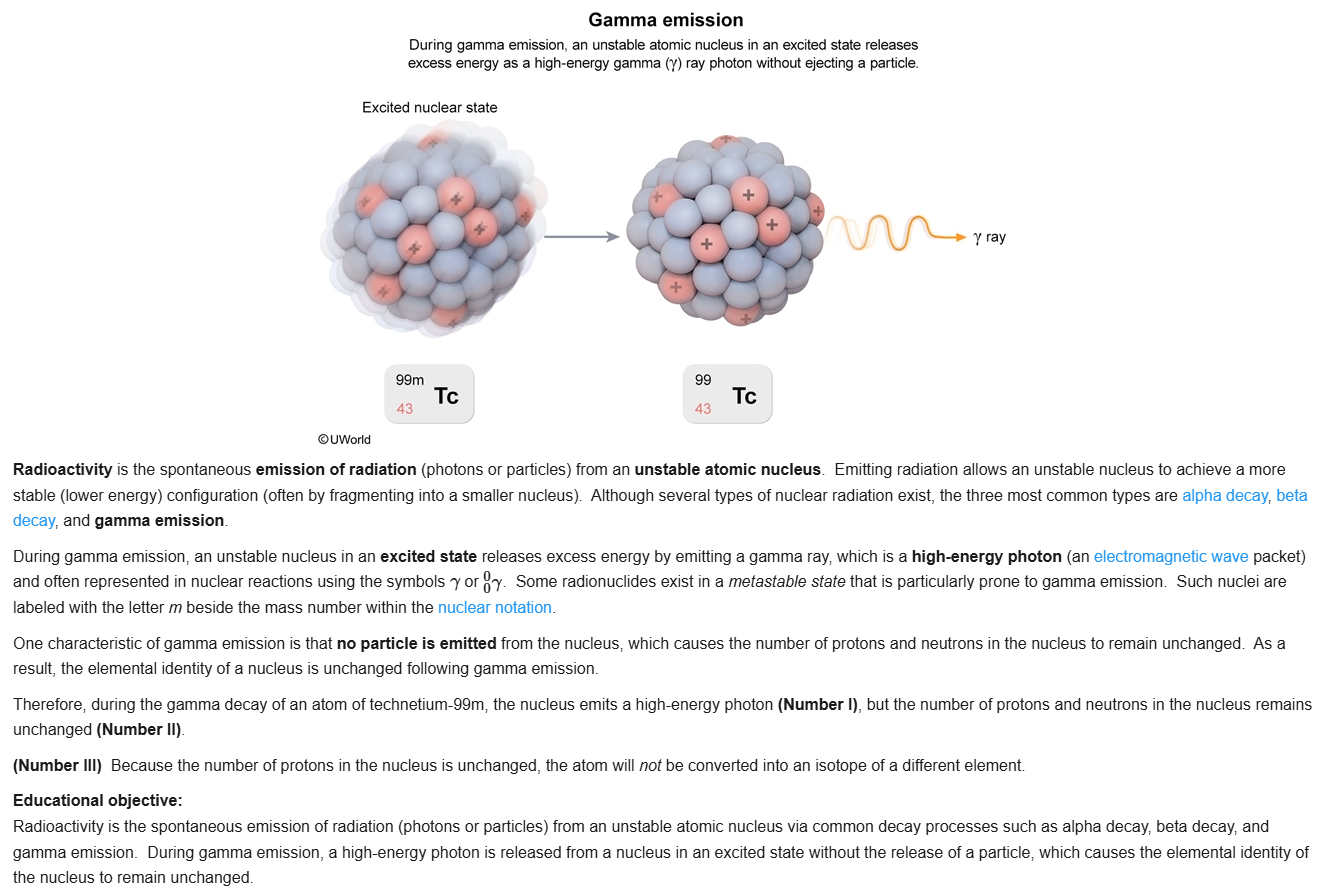
4
New cards
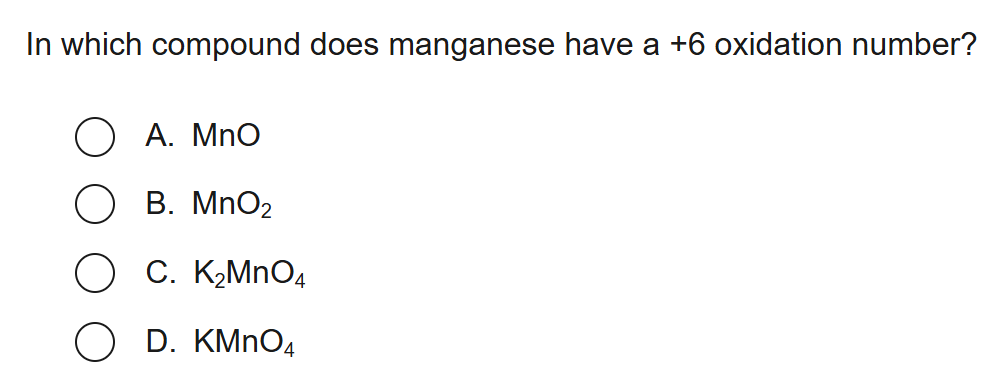
C
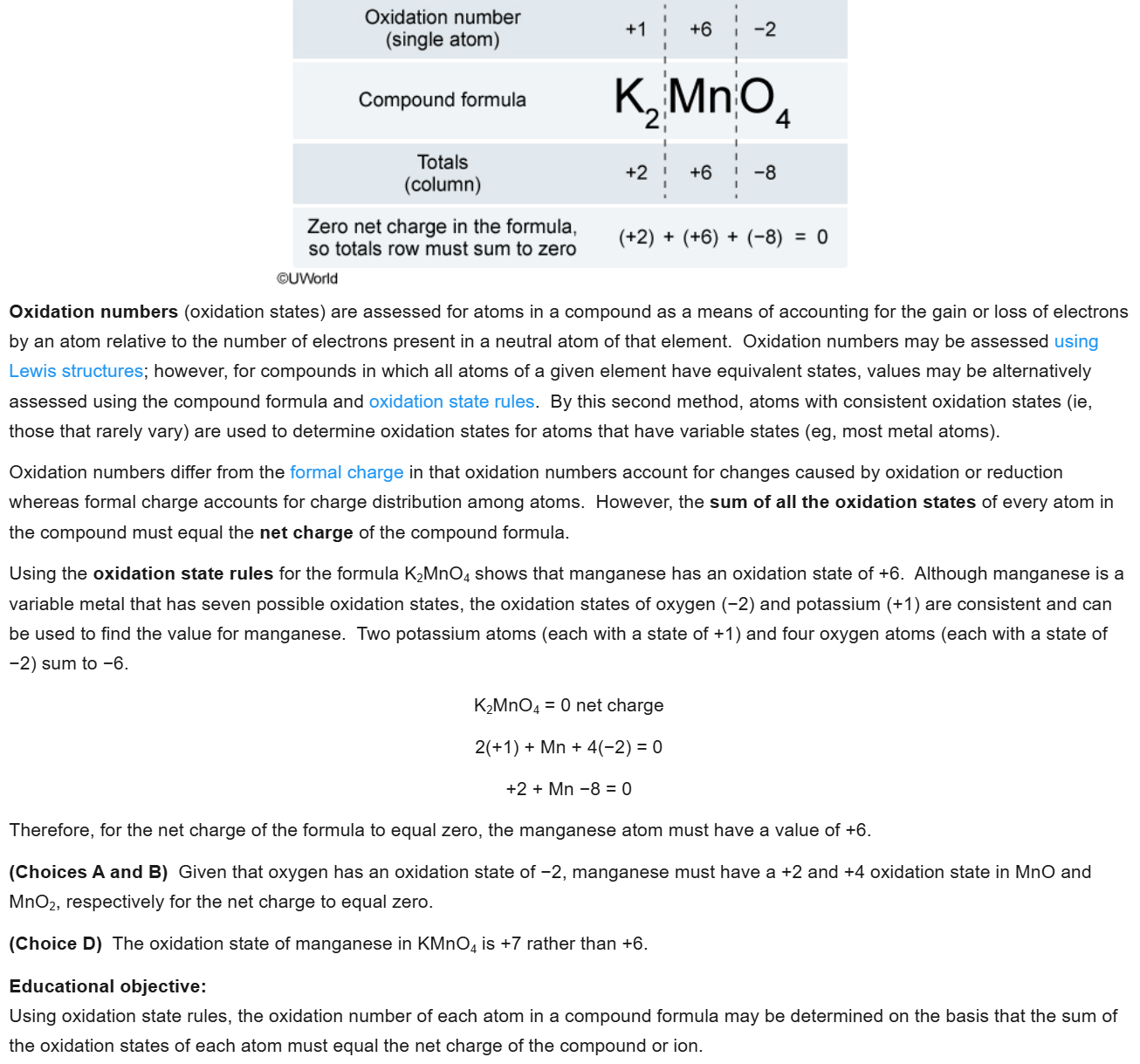
5
New cards
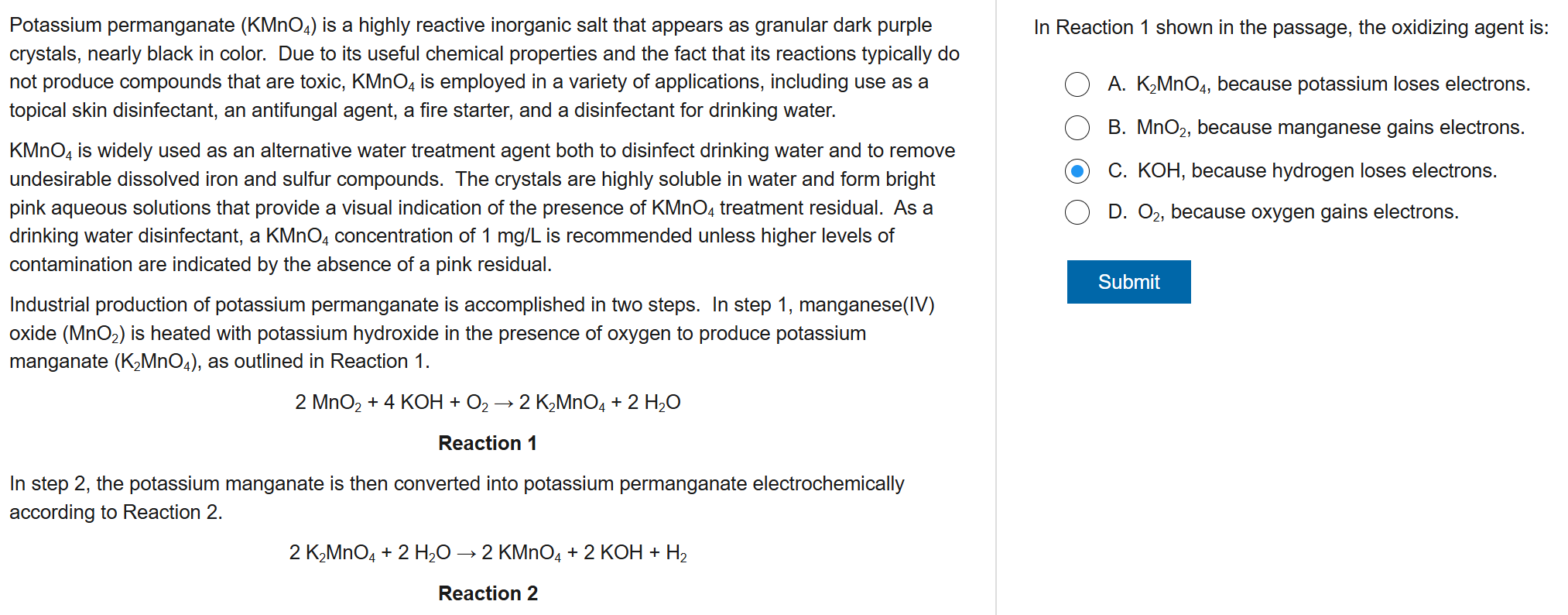
D
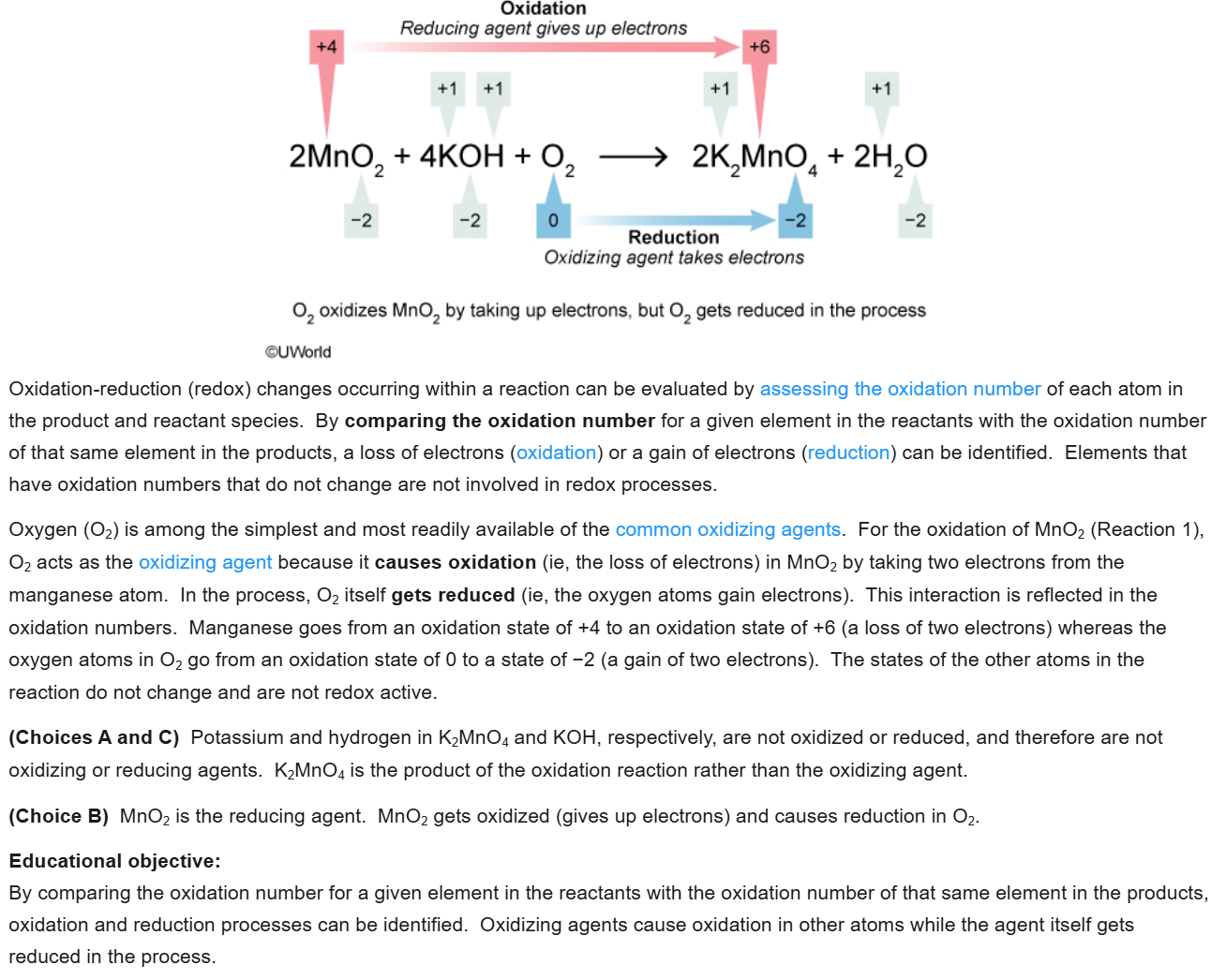
6
New cards
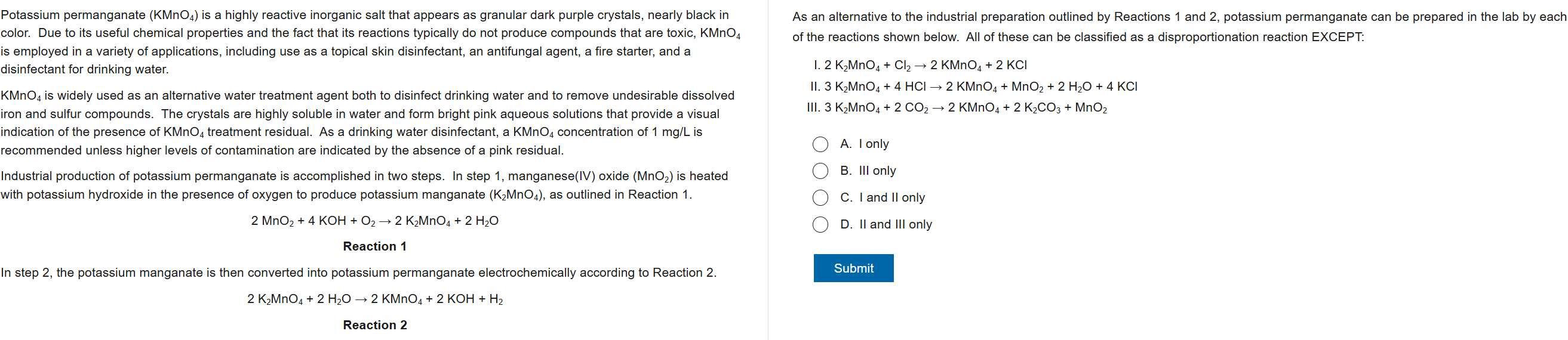
A

7
New cards

What volume of H2 gas will be generated in Reaction 2 described in the passage if 10.00 L of a 5.00 M solution of K2MnO4 react at STP?
a) 1.12 L
b) 22.4 L
c) 560 L
d) 1120 L
C

8
New cards

B

9
New cards
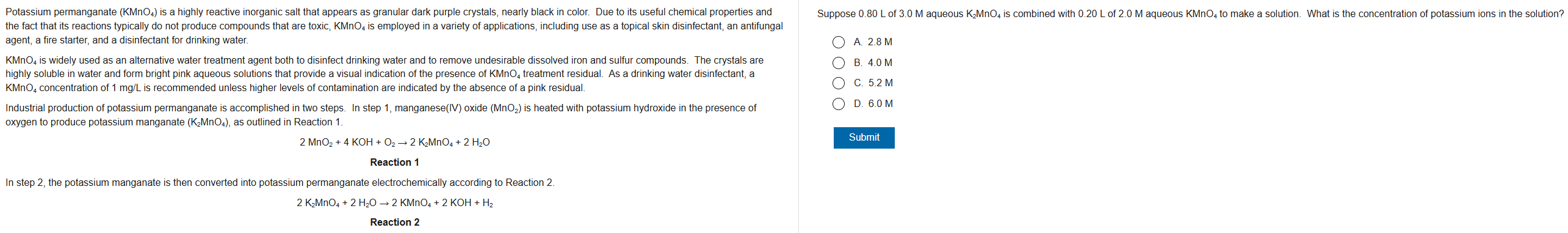
C
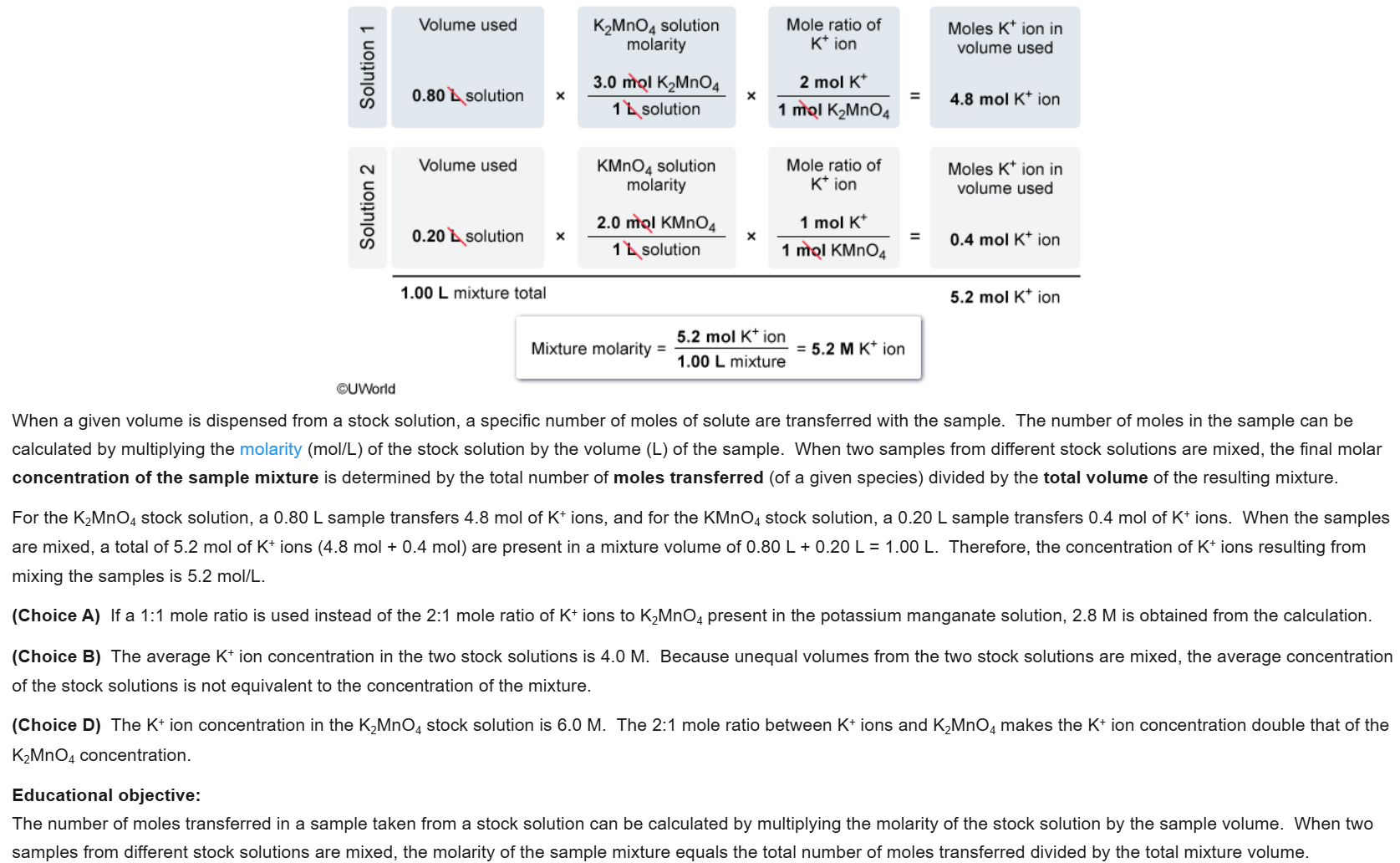
10
New cards
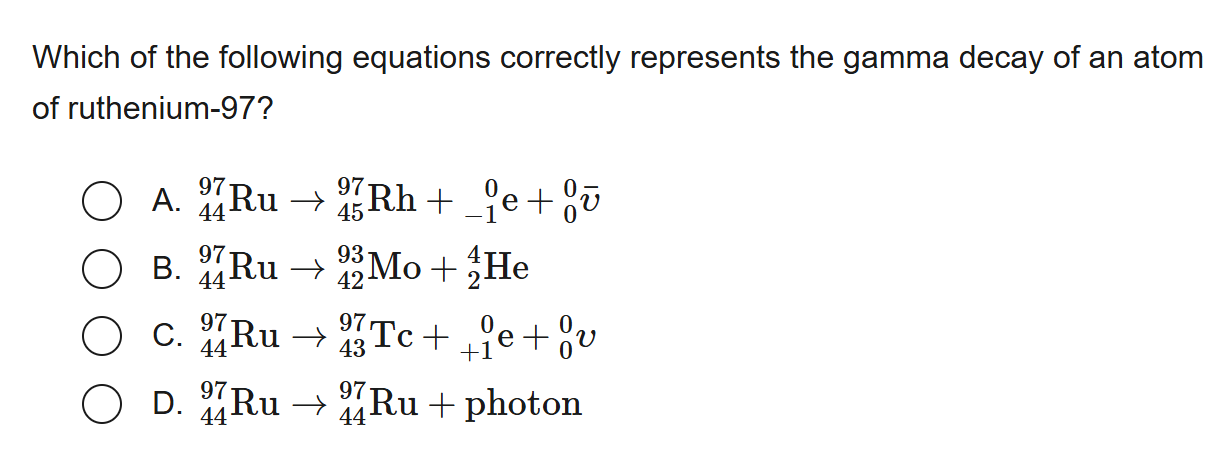
D
11
New cards
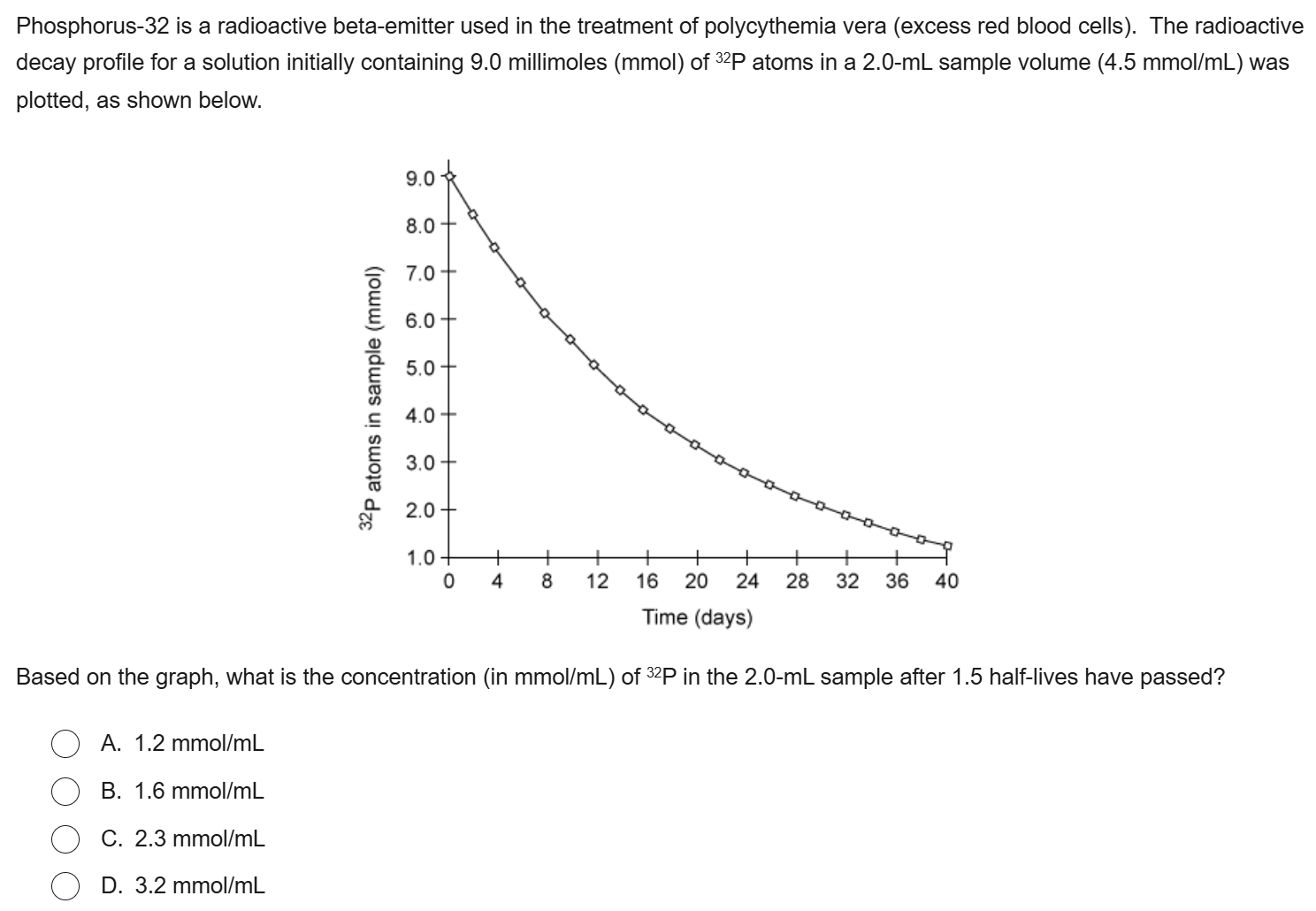
B
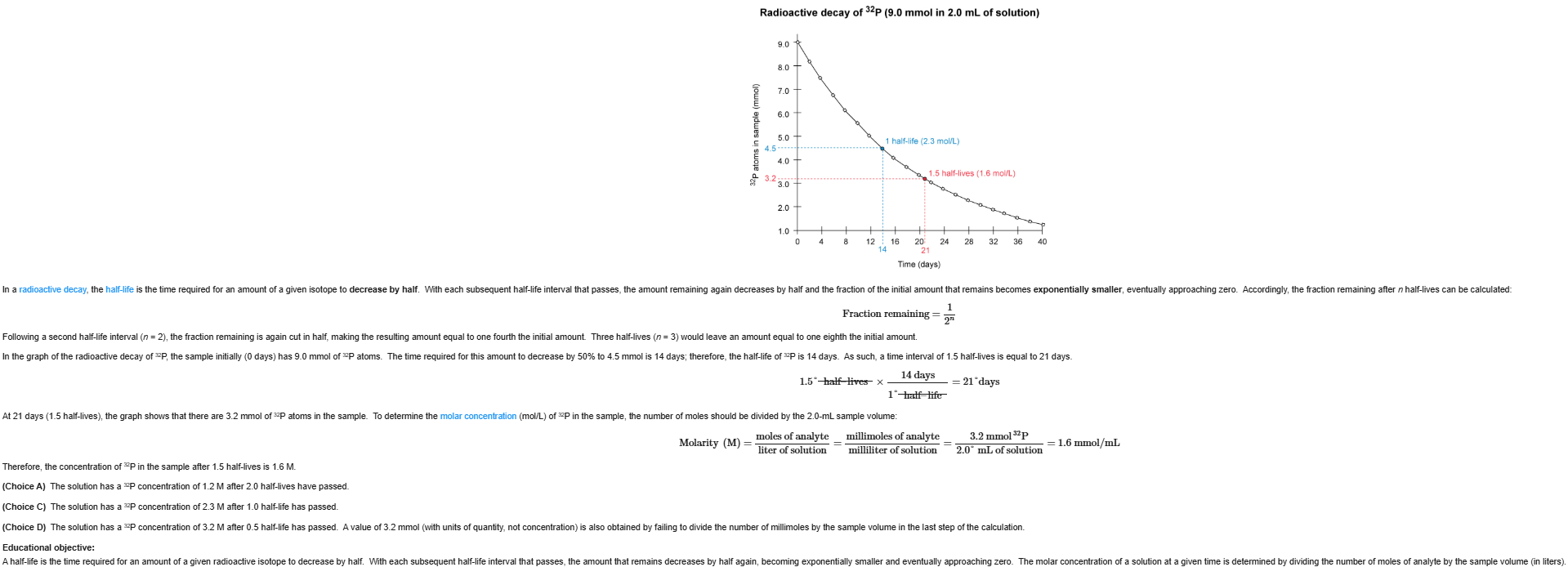
12
New cards

A) I and II only
B) I and III only
C) II and III only
D) I, II, and III
A
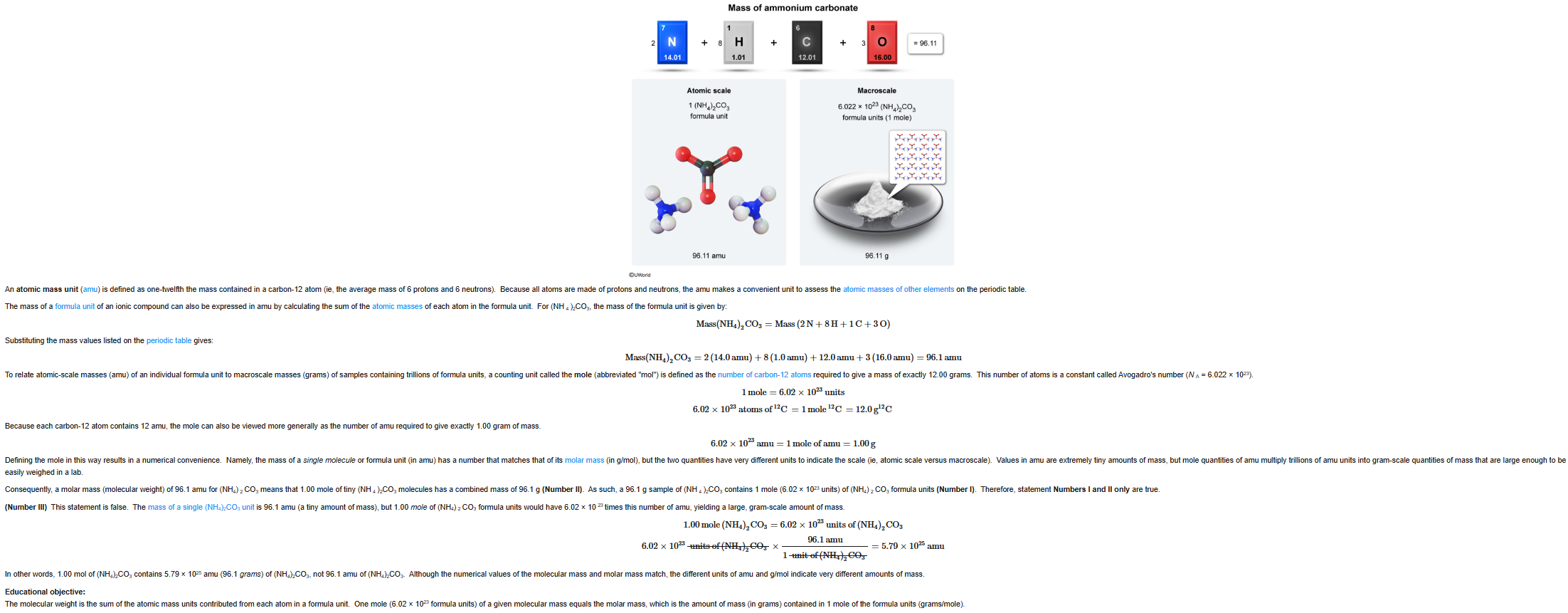
13
New cards
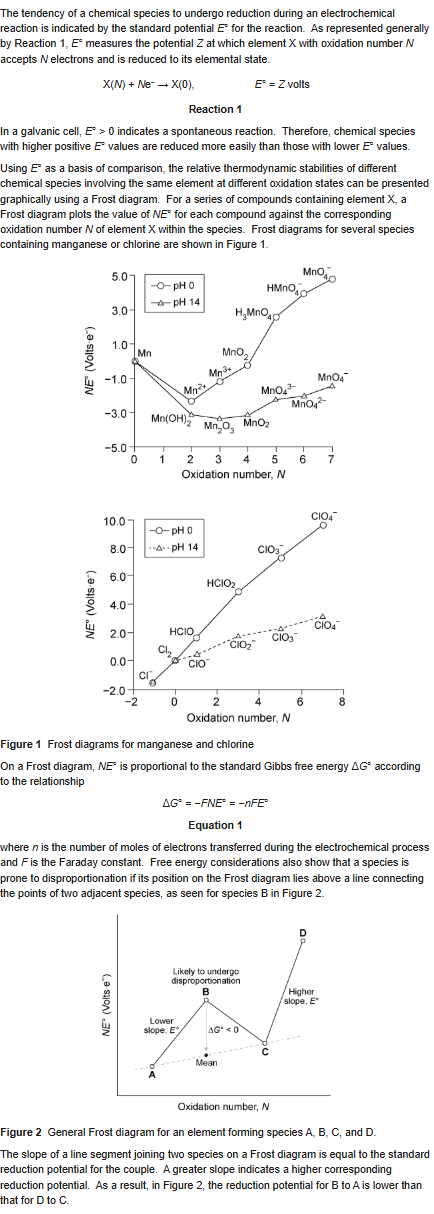
Based on the passage, which of the following species is LEAST likely to undergo a disproportionation reaction?
A) H3MnO4, pH = 0
B) MnO43-, pH = 14
C) Mn3+, pH = 0
D) MnO2, pH = 14
D
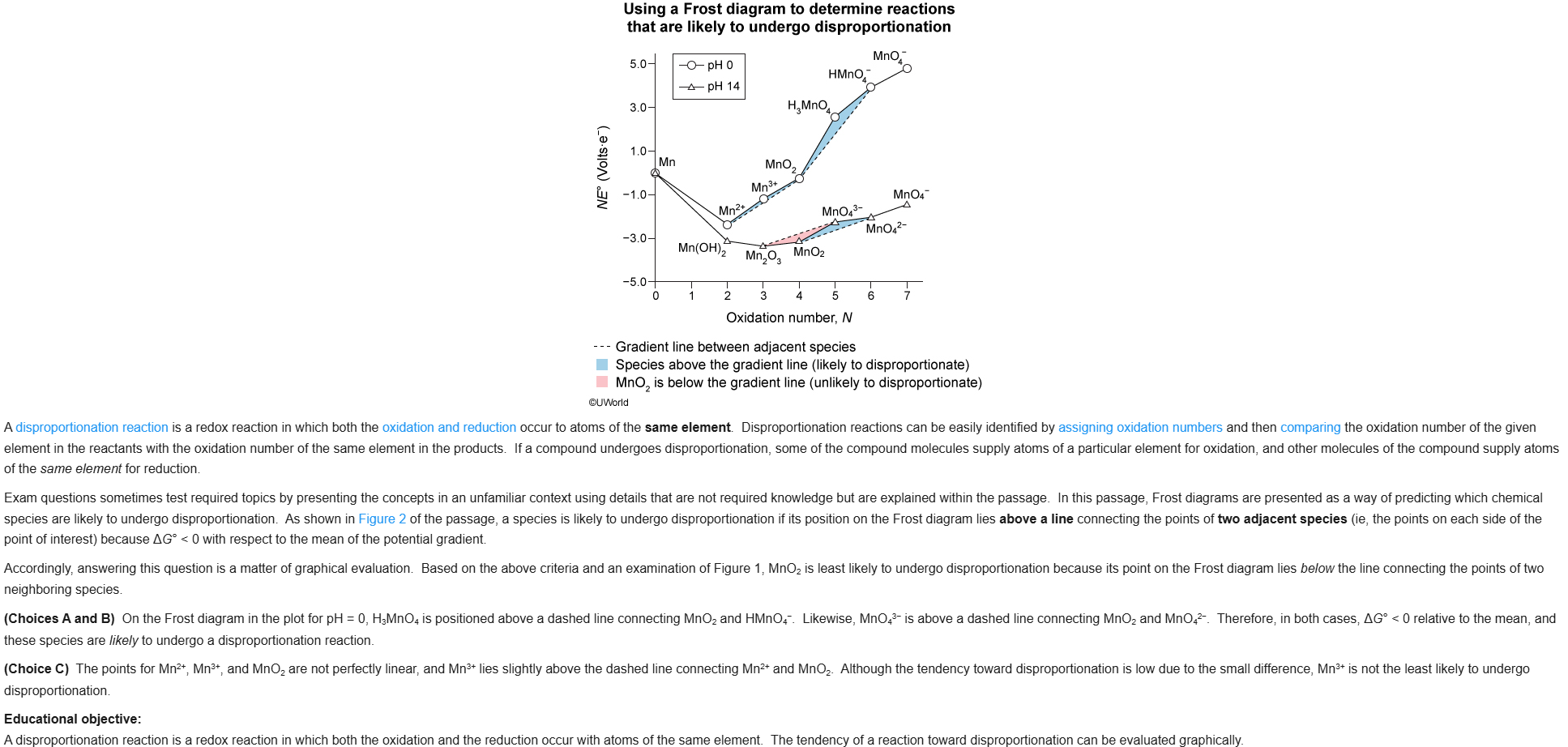
14
New cards
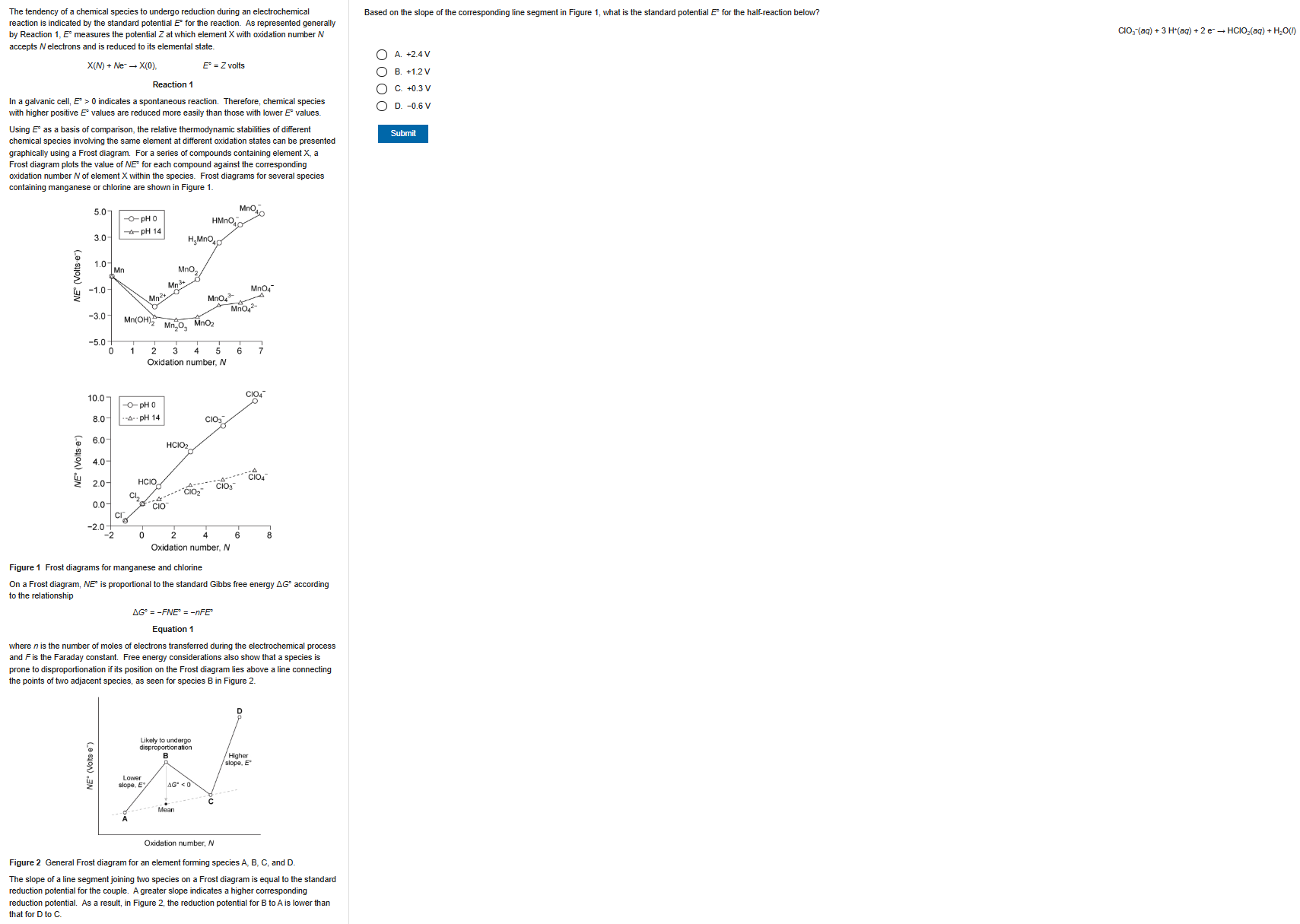
B
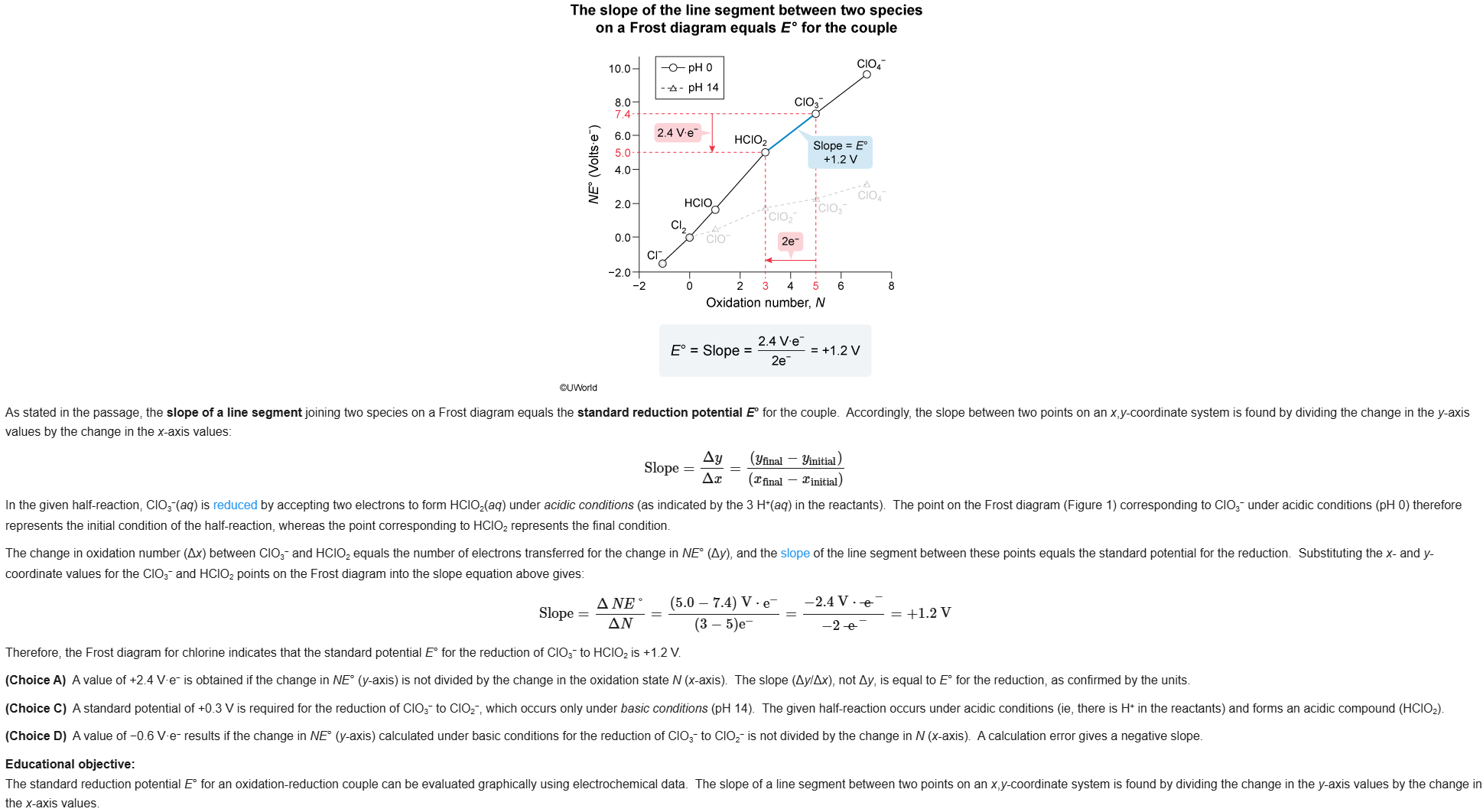
15
New cards
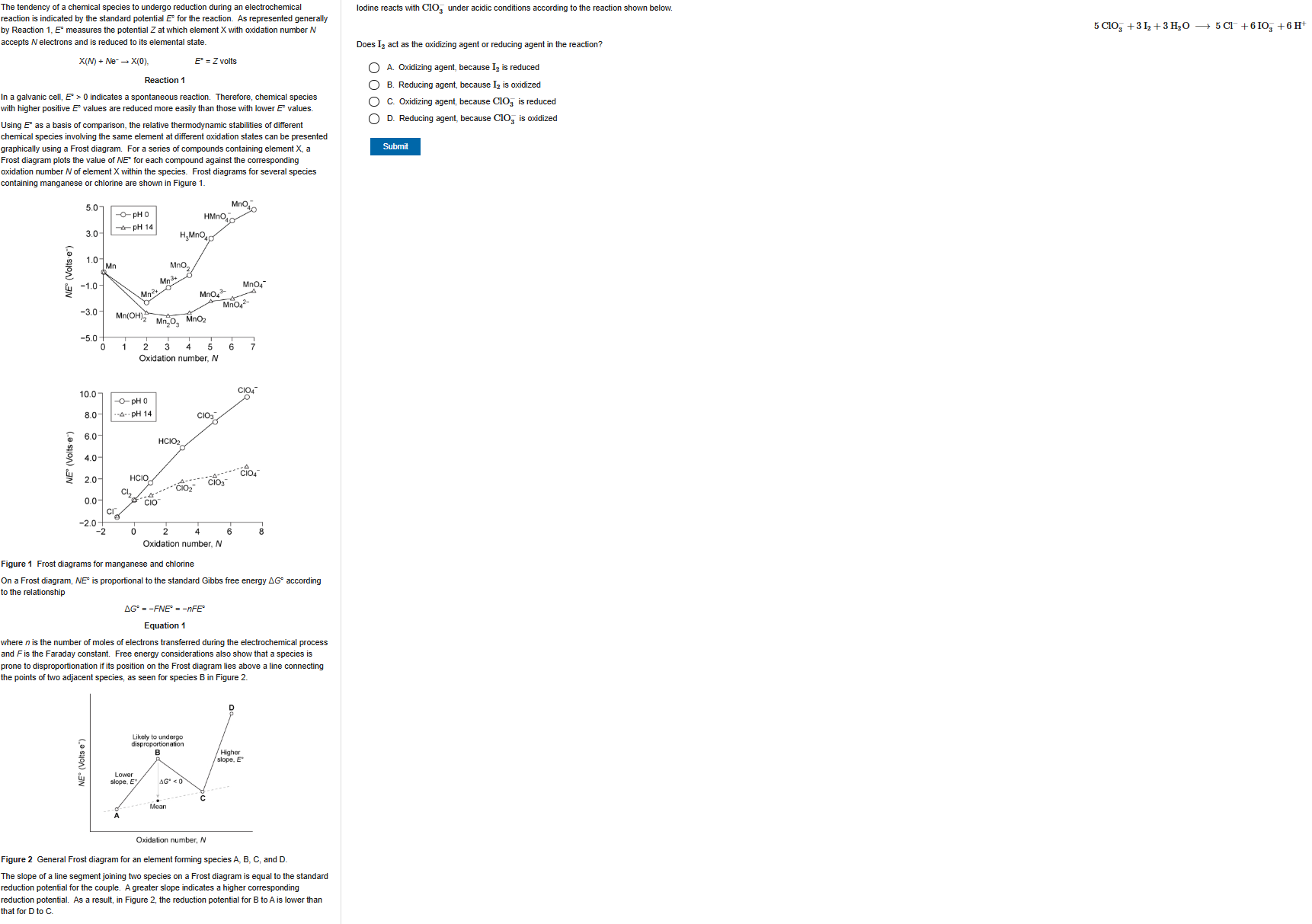
B

16
New cards
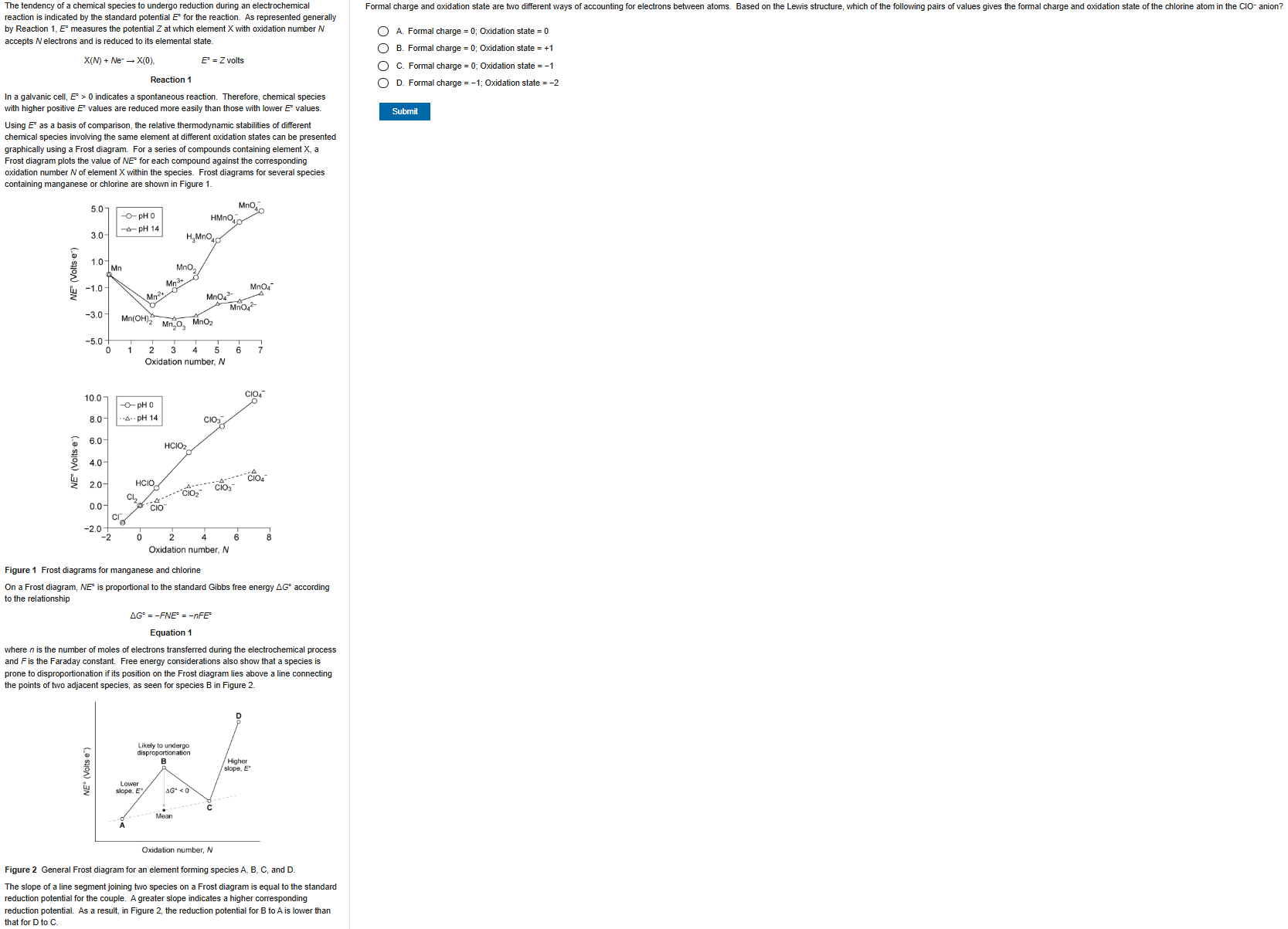
B

17
New cards

A

18
New cards

A
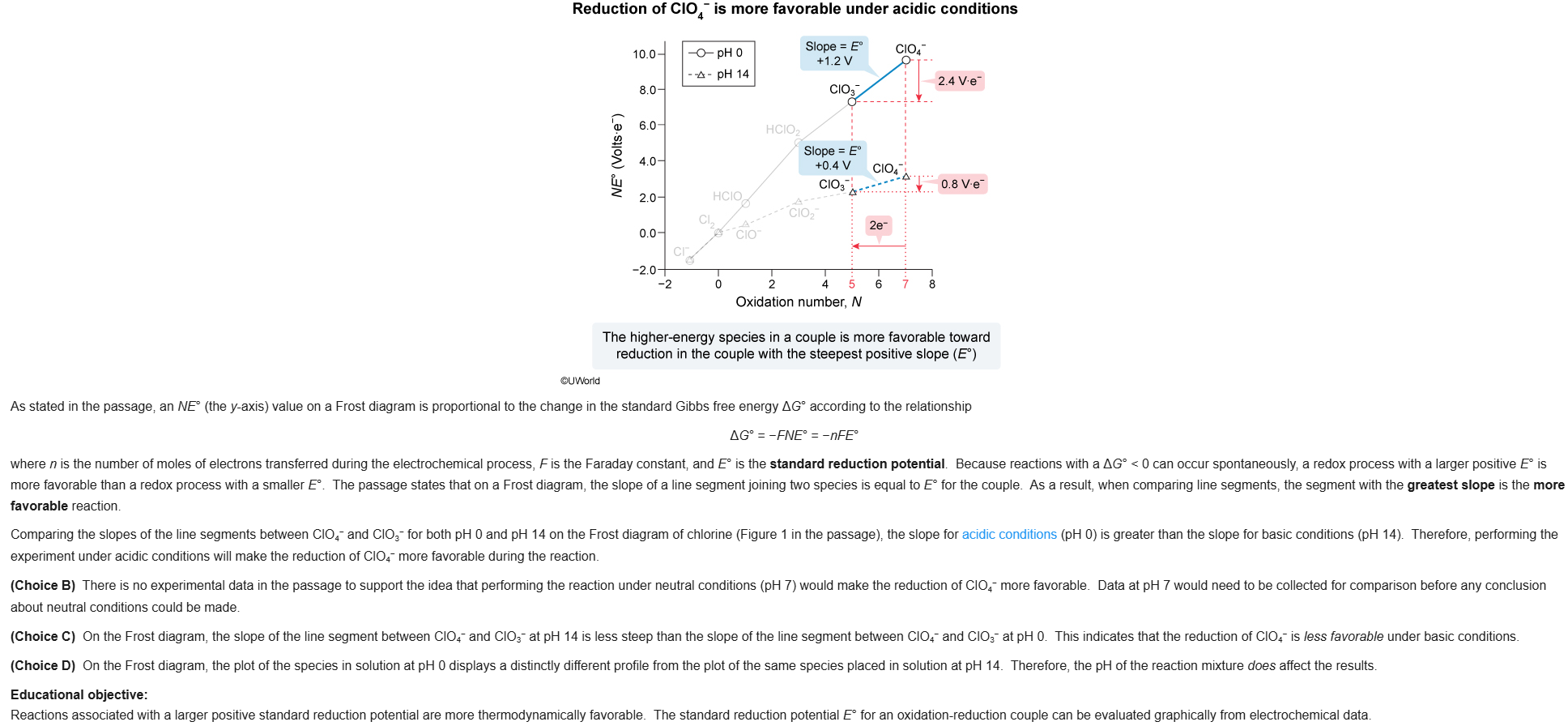
19
New cards
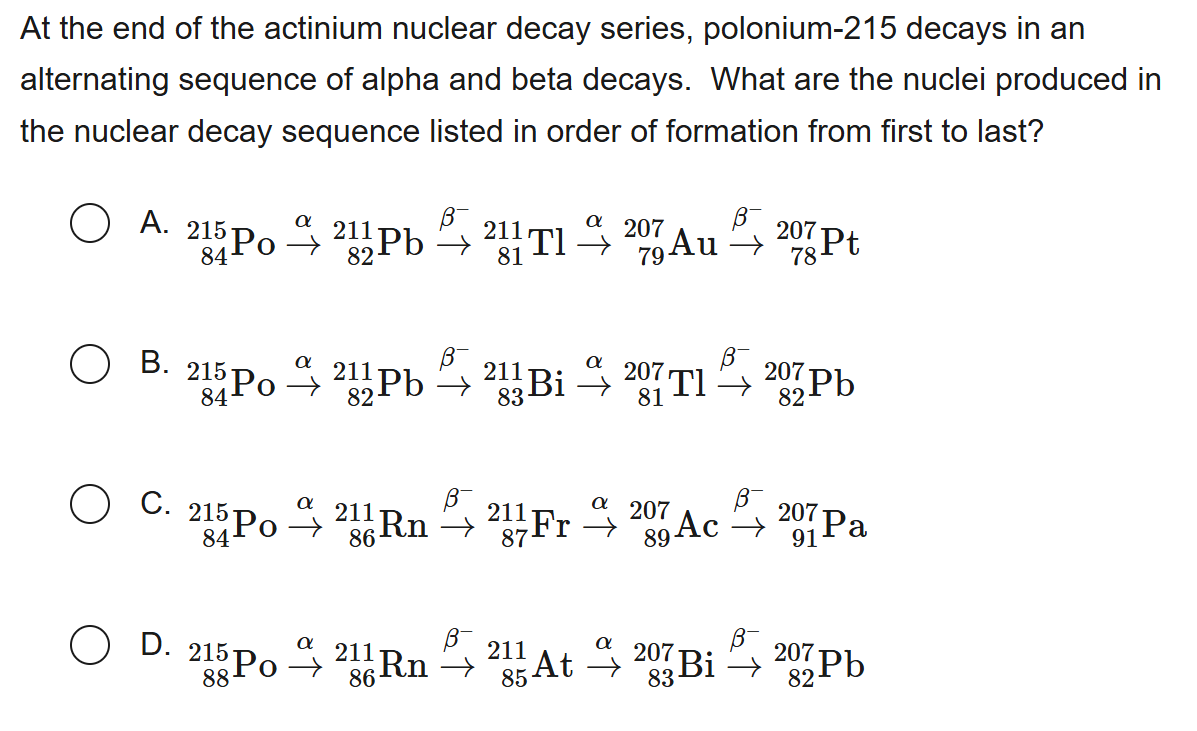
B

20
New cards
B
21
New cards
C
22
New cards
C
23
New cards
C
24
New cards
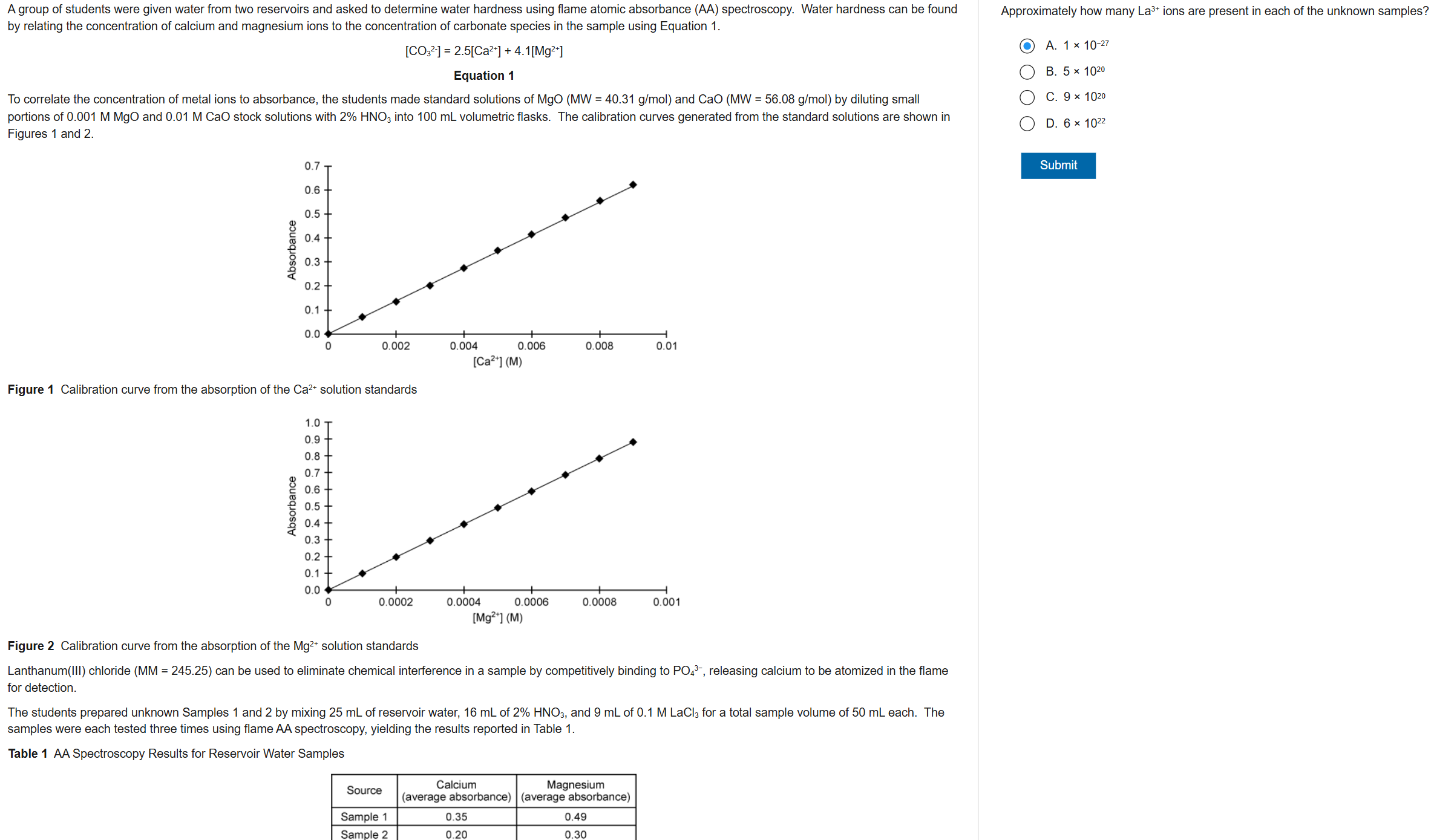
B

25
New cards
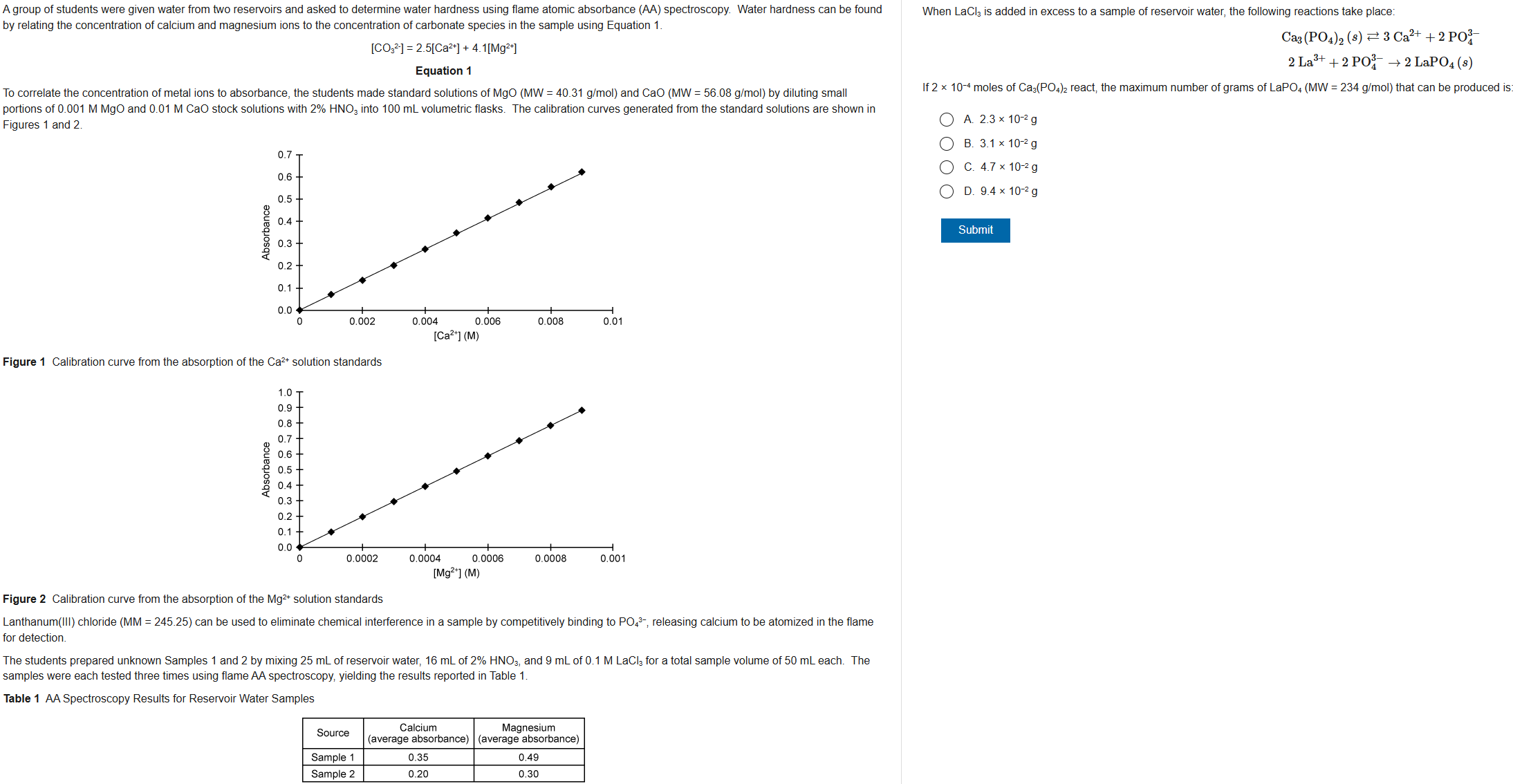
D

26
New cards
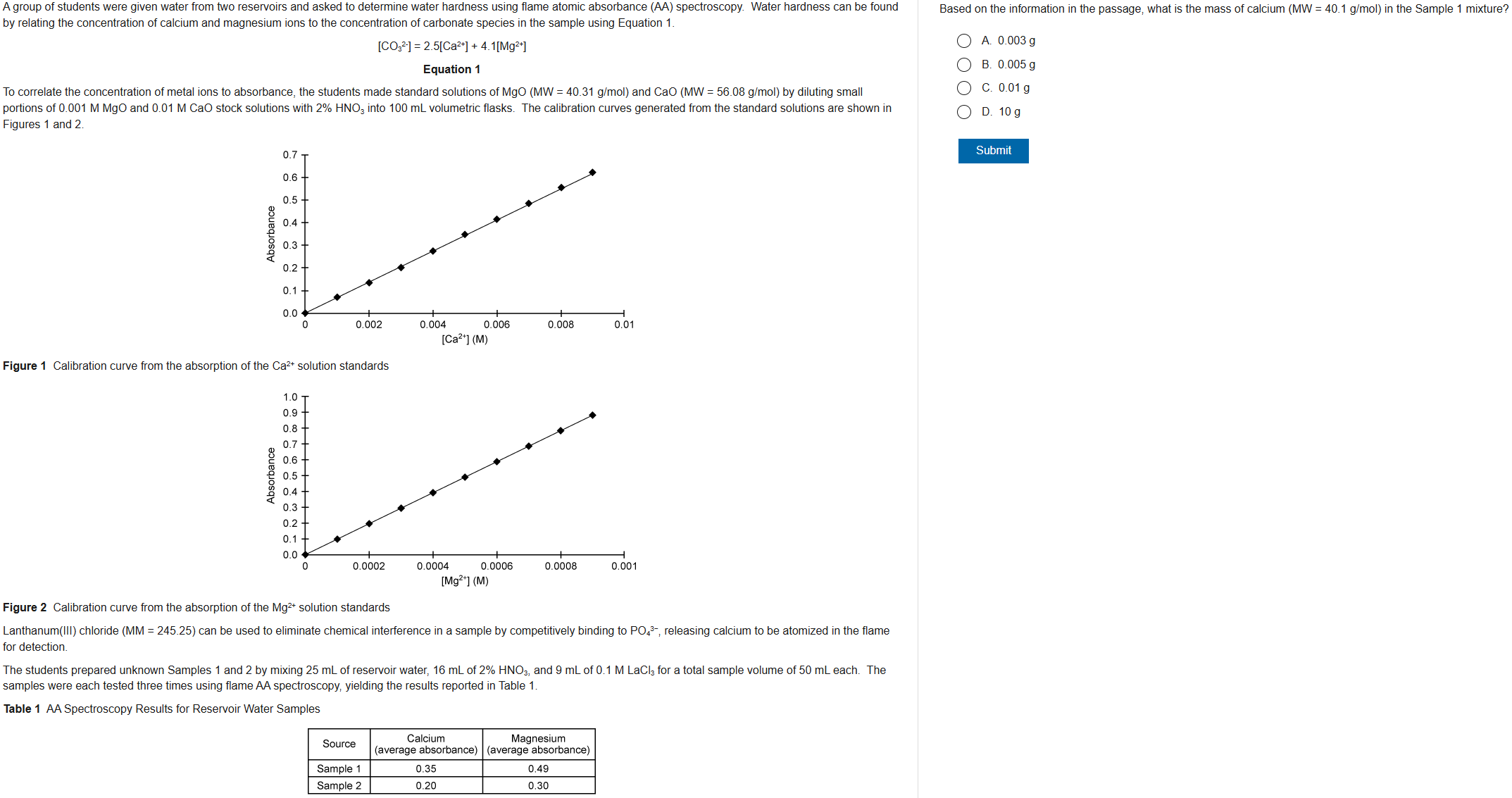
C
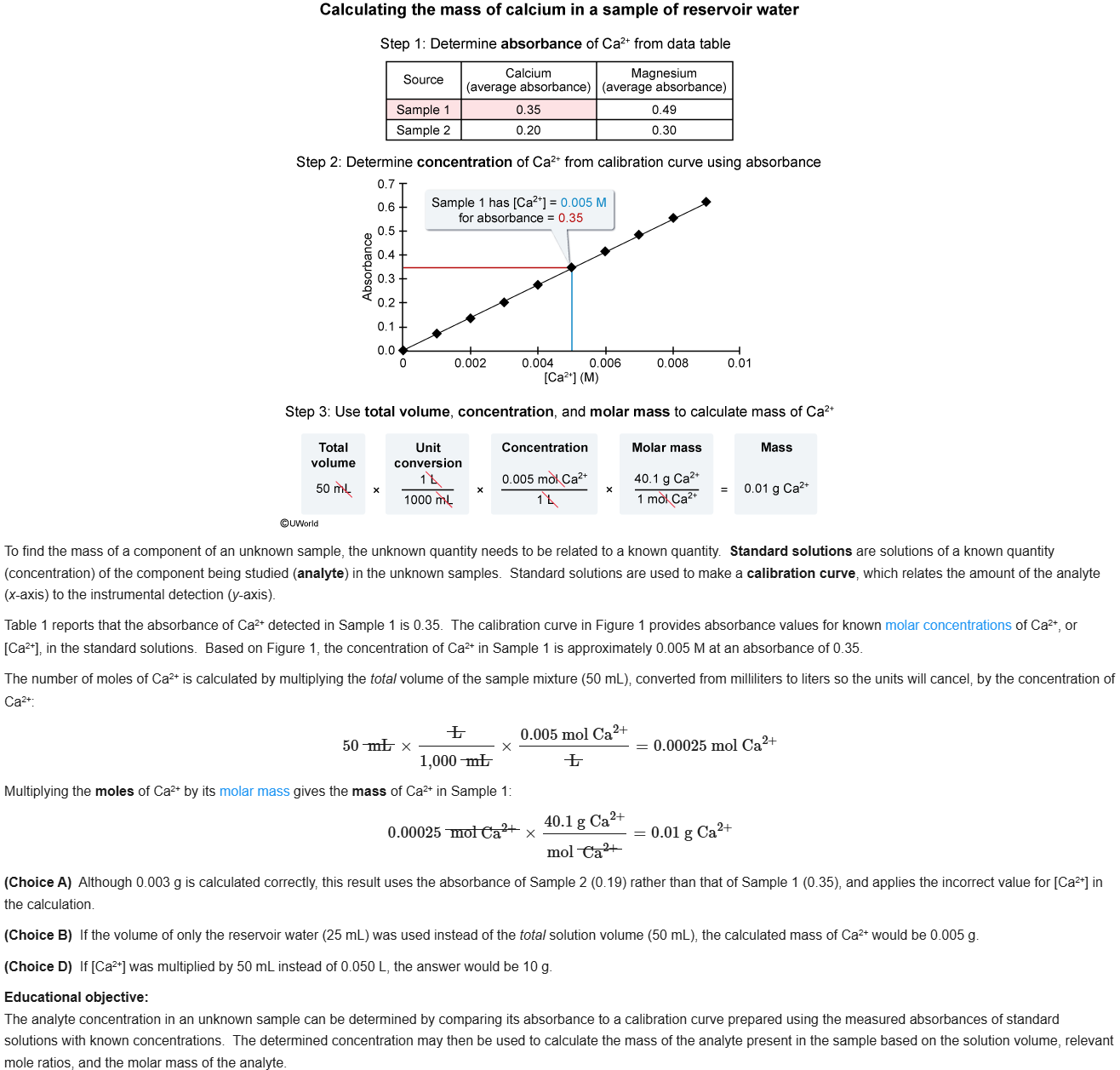
27
New cards
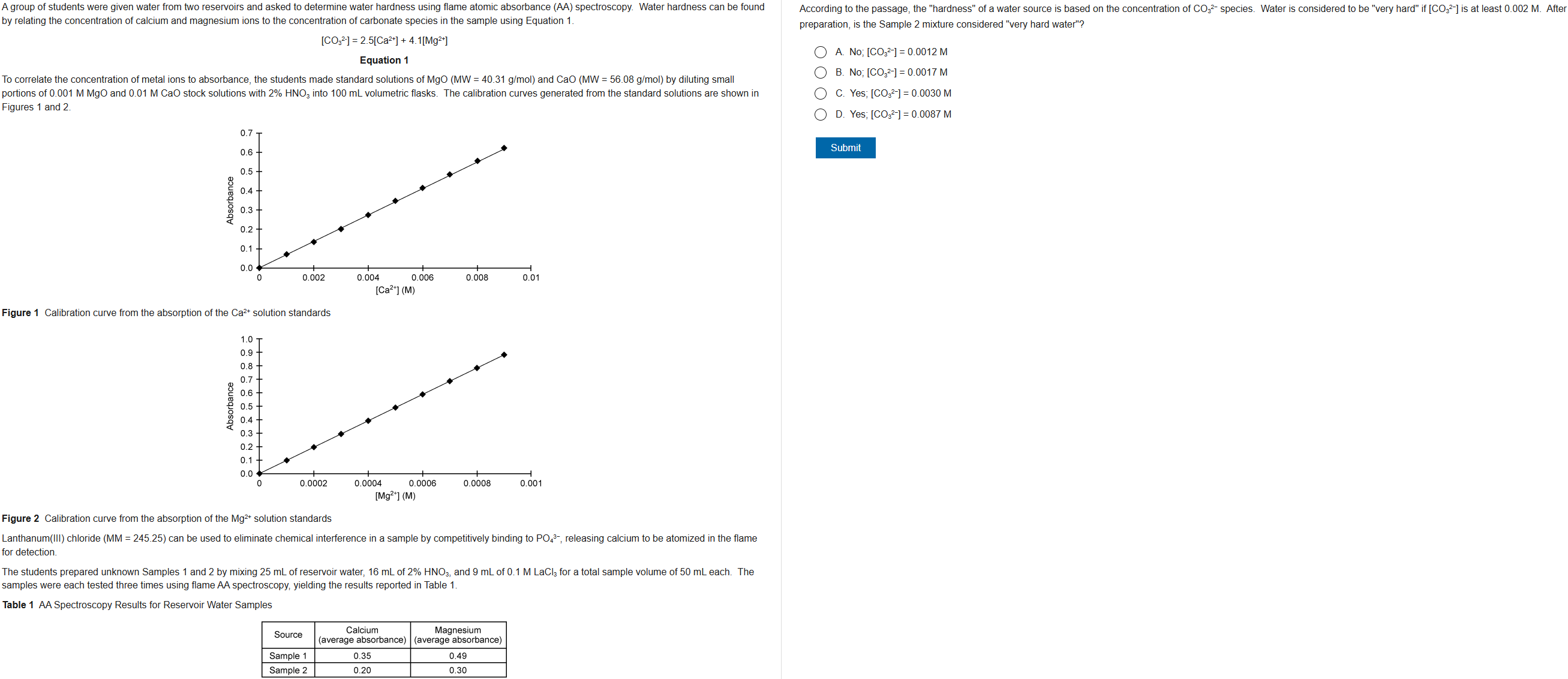
D
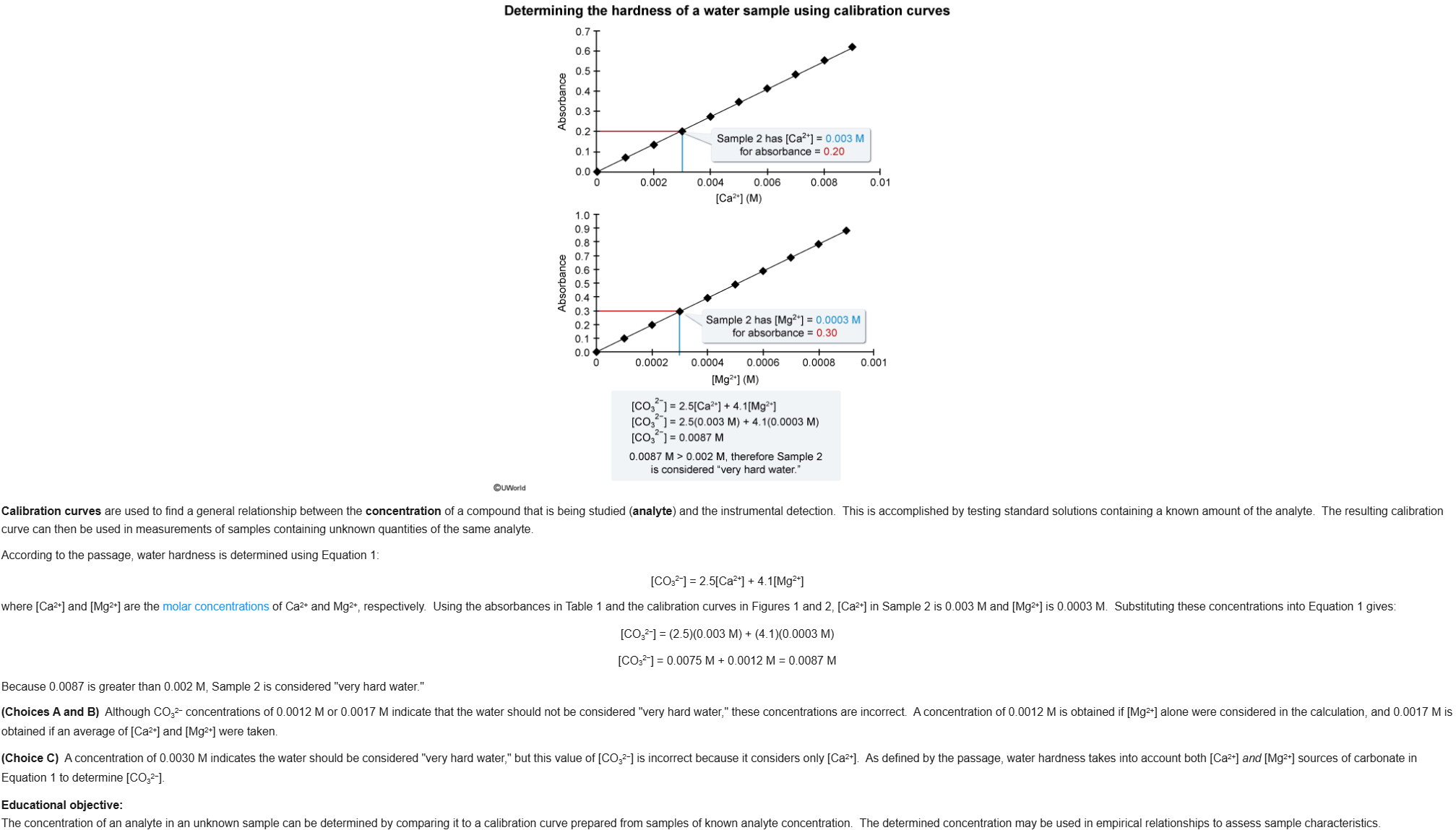
28
New cards

B
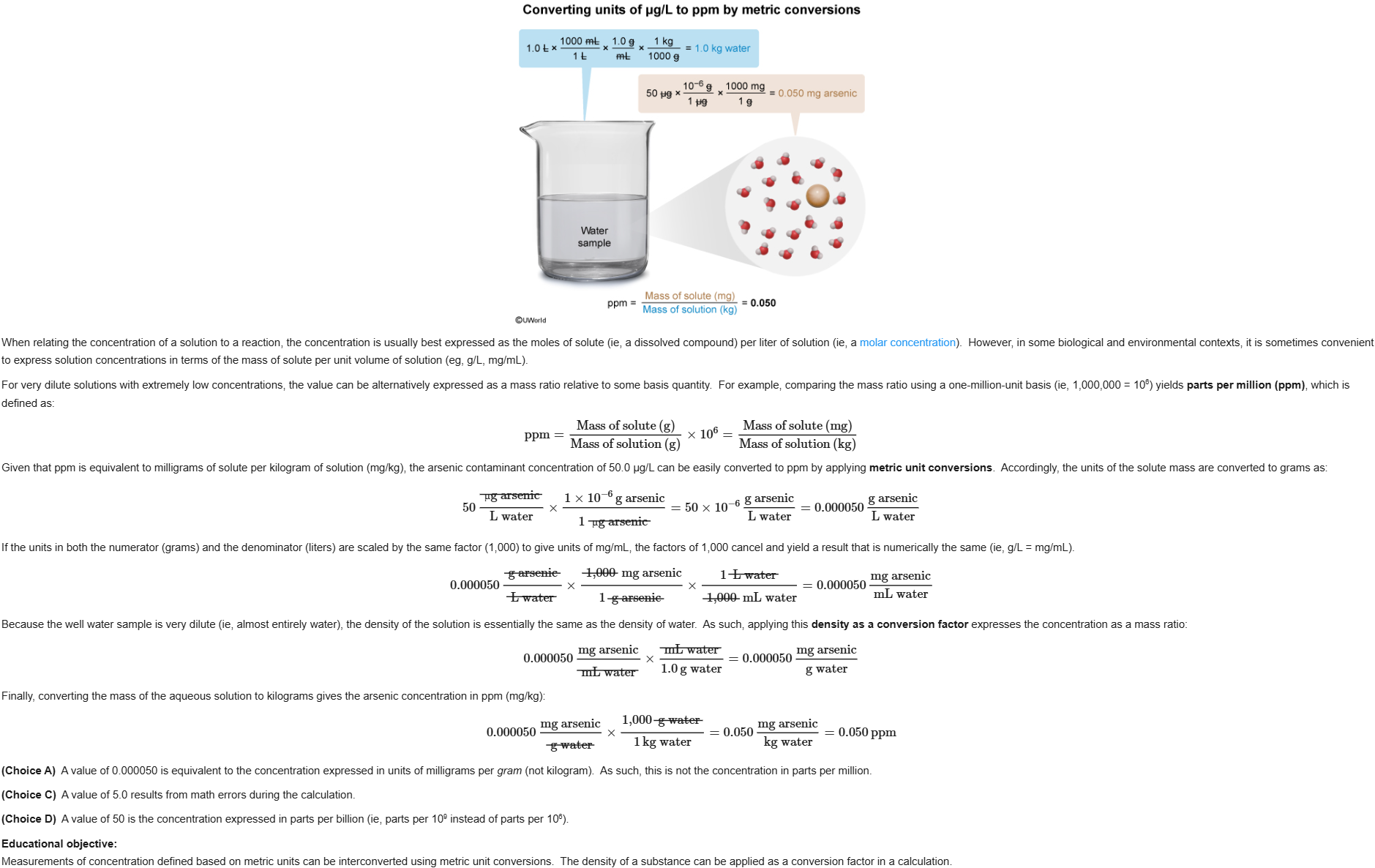
29
New cards
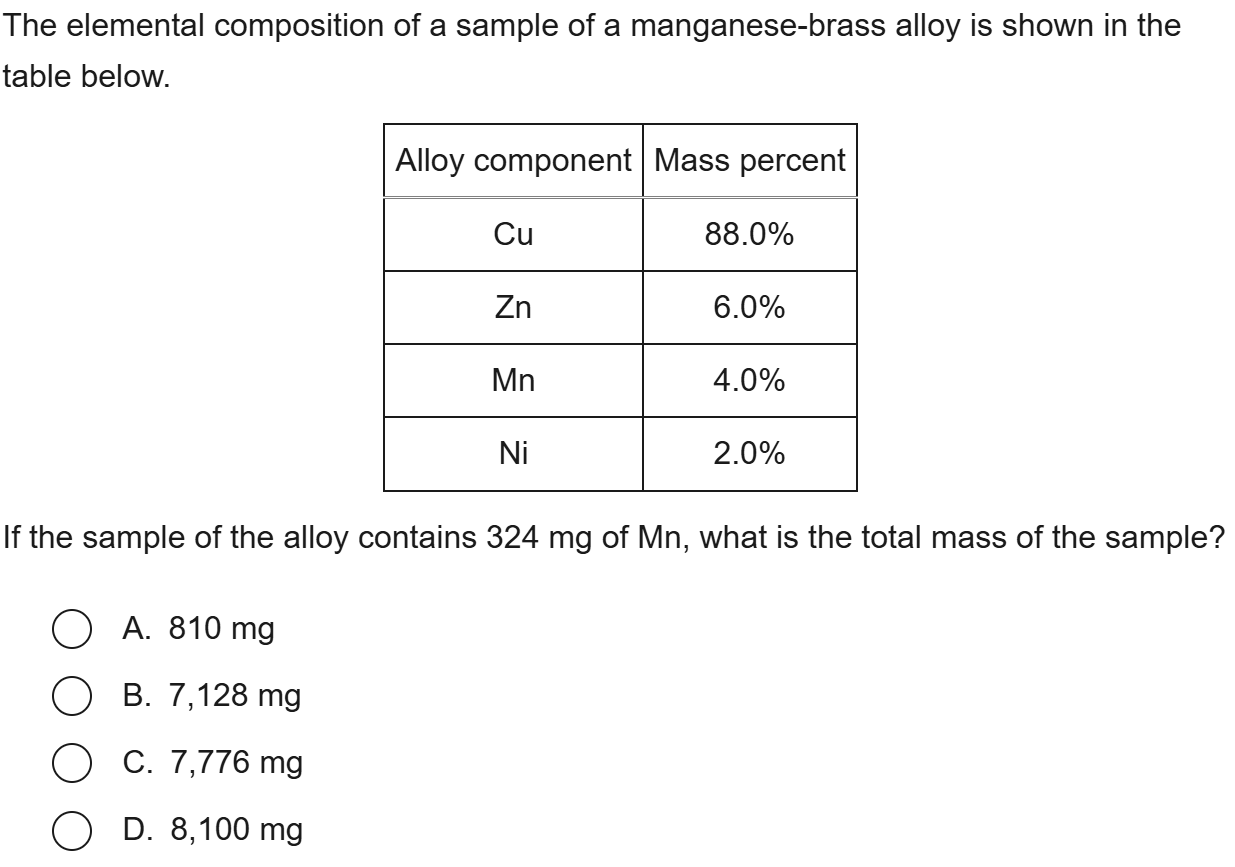
D
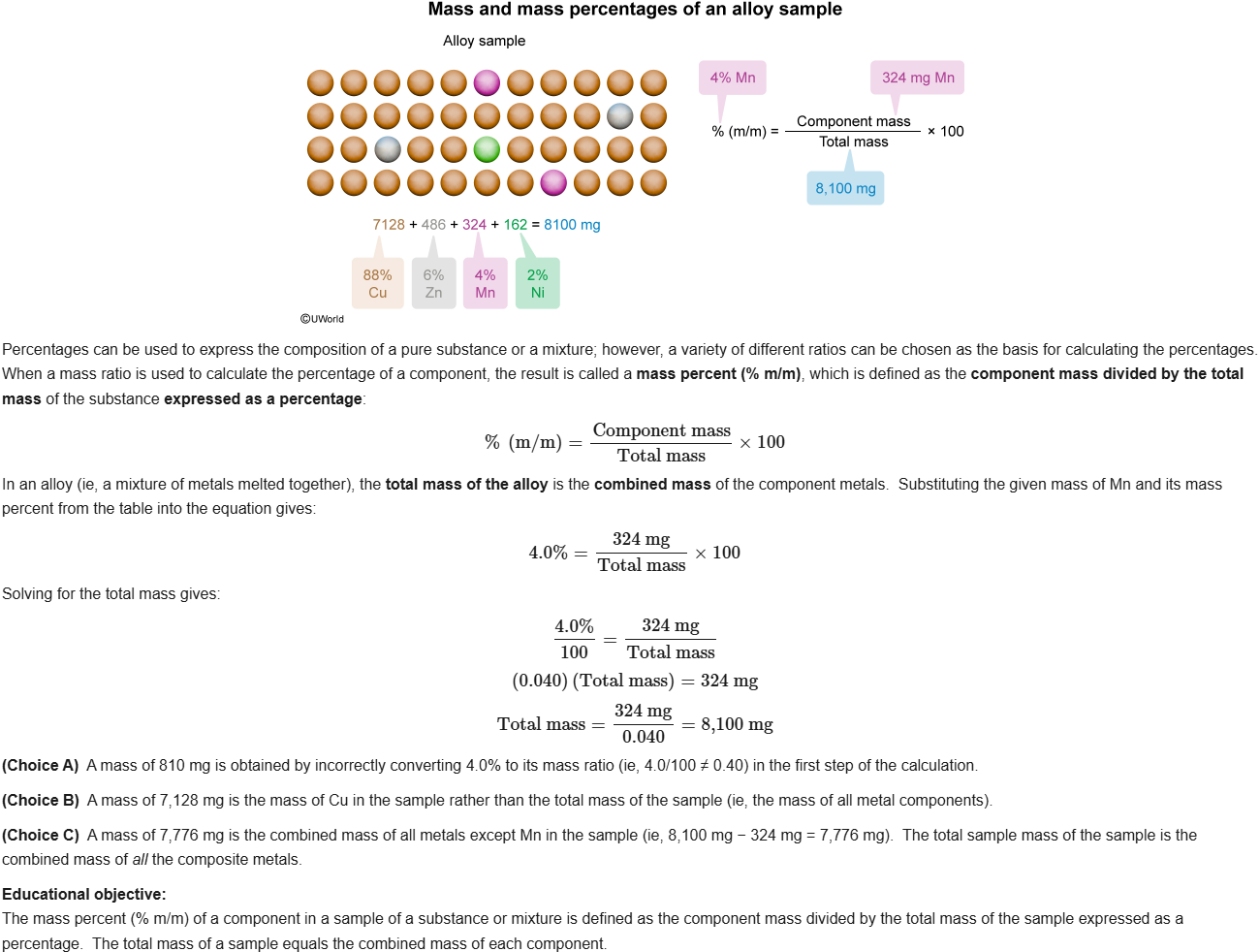
30
New cards

A
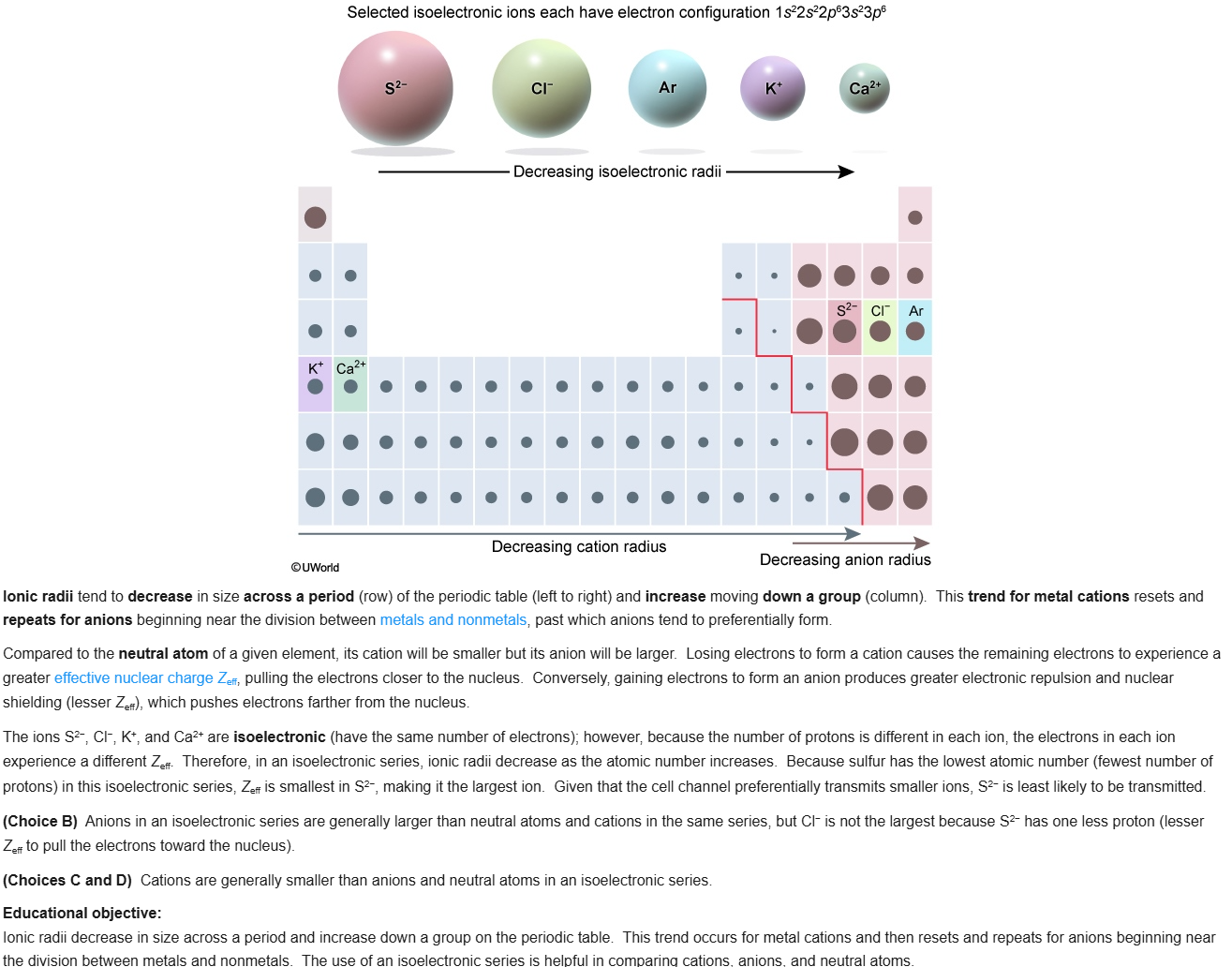
31
New cards
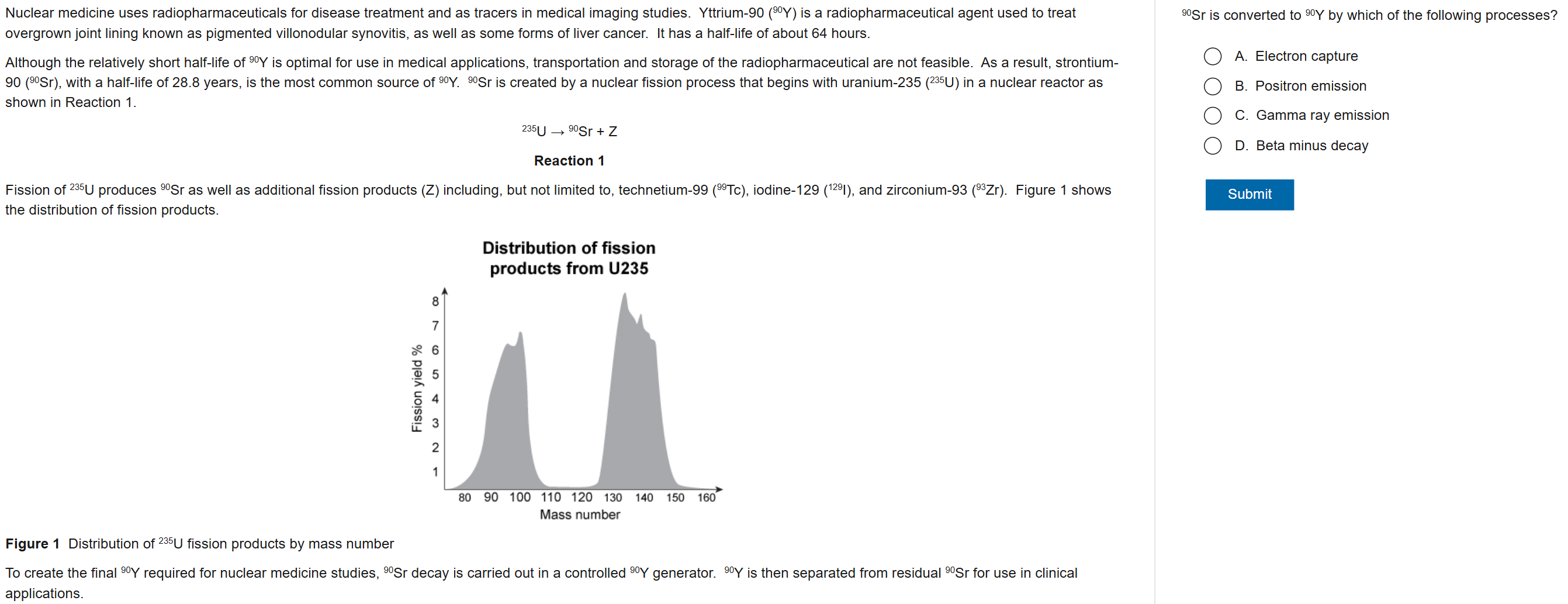
D
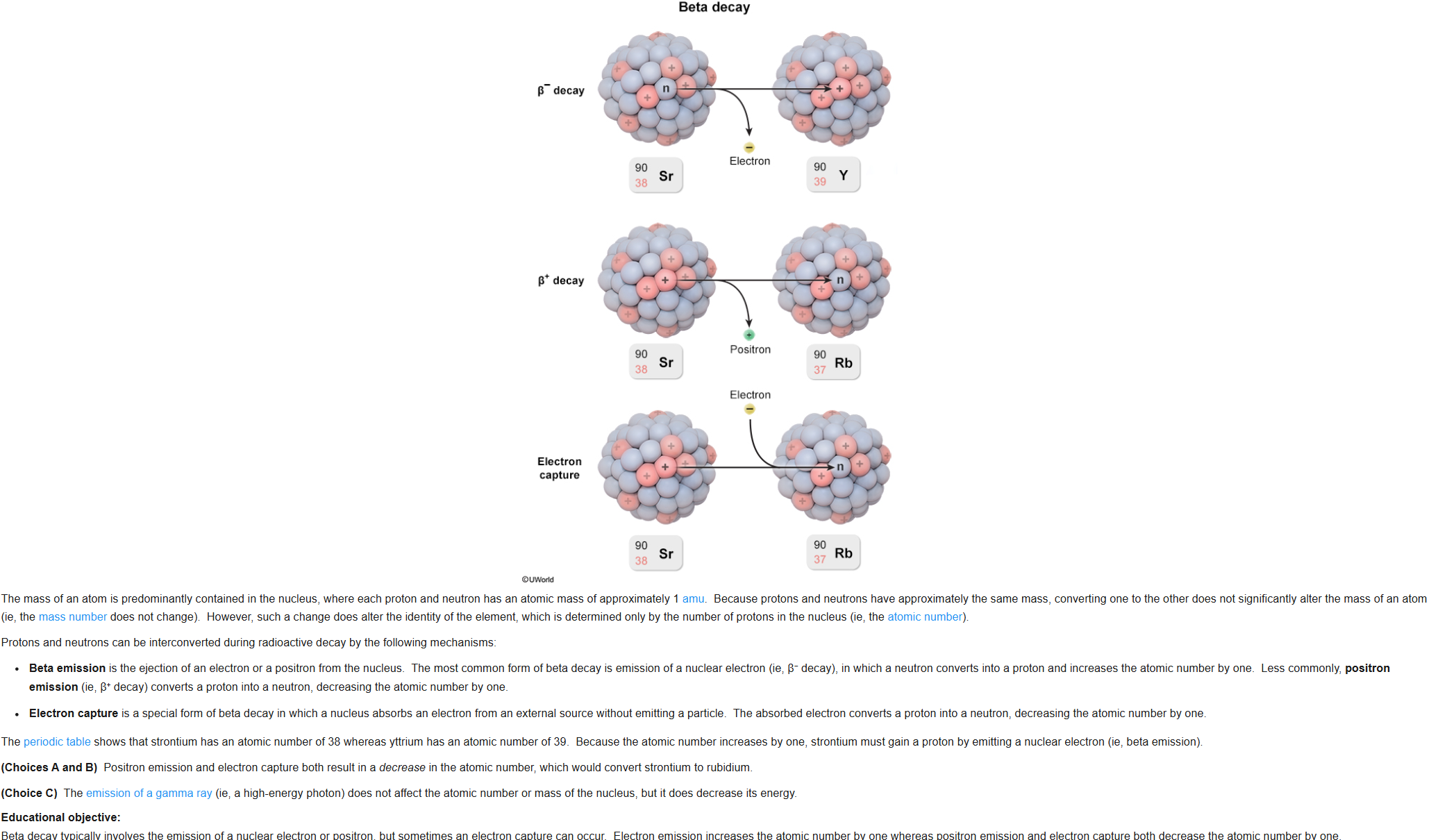
32
New cards
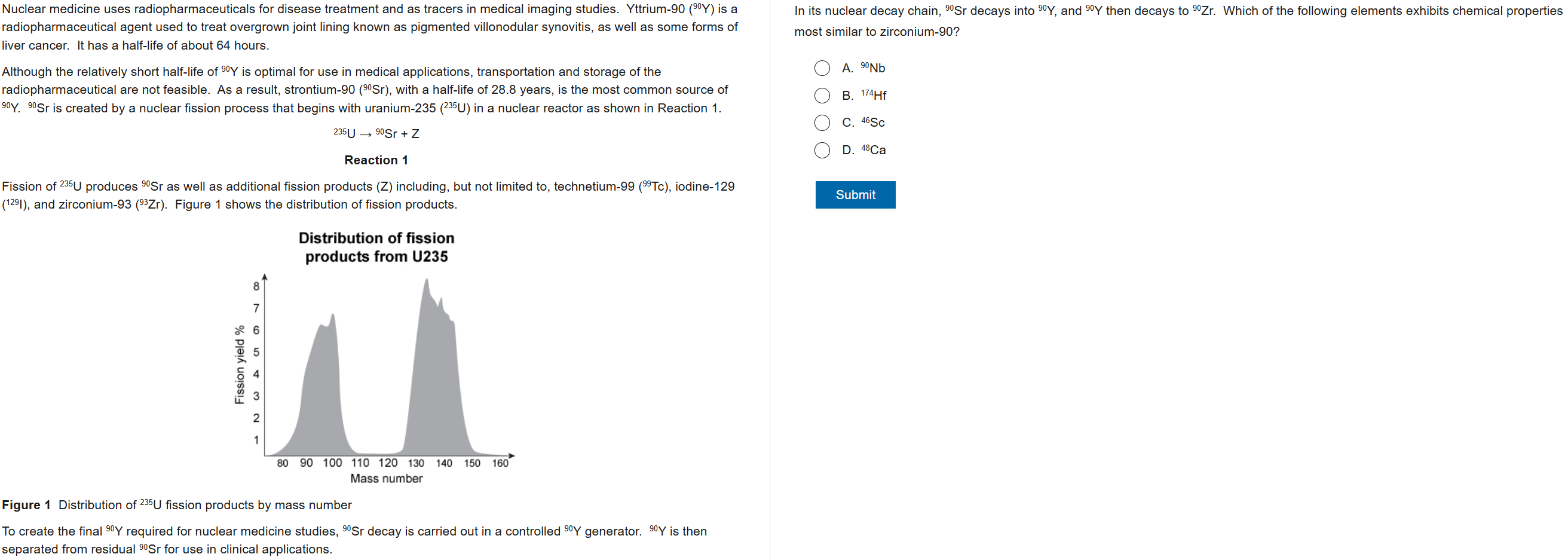
B

33
New cards
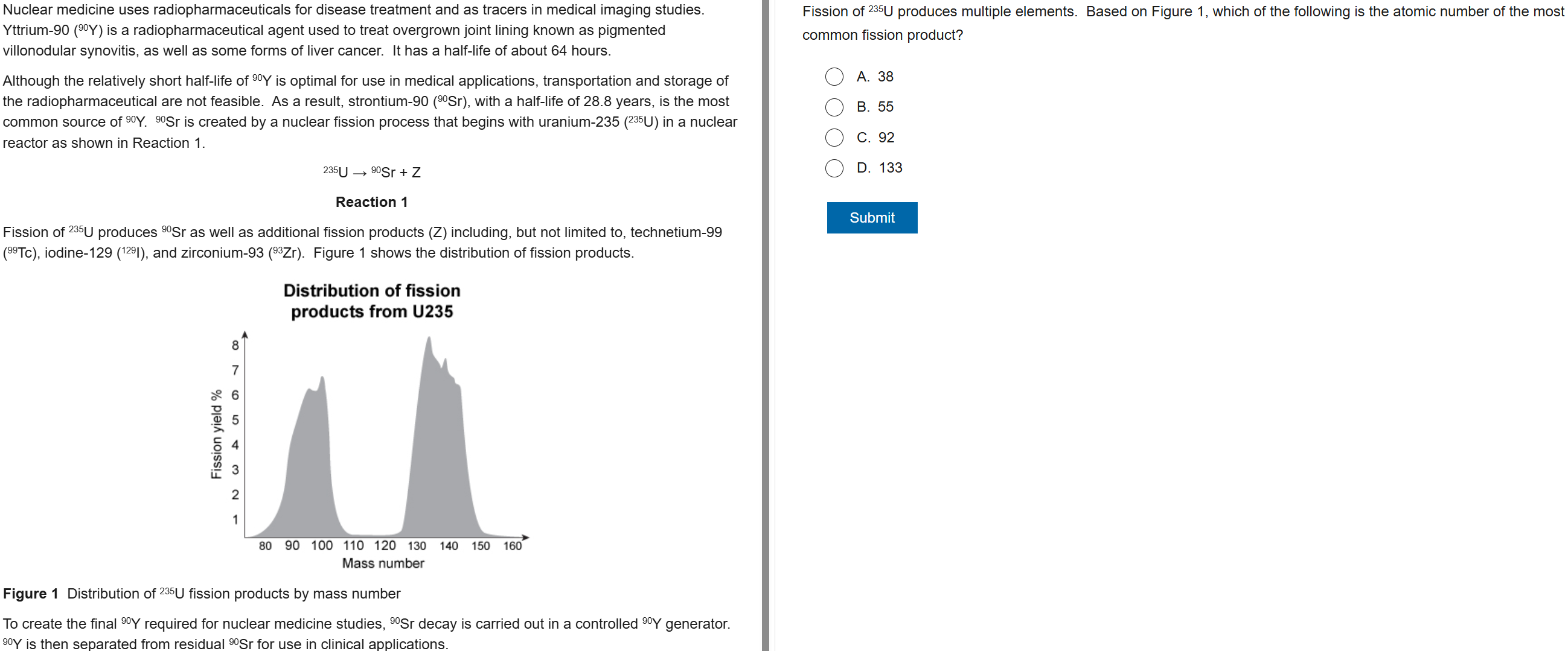
B
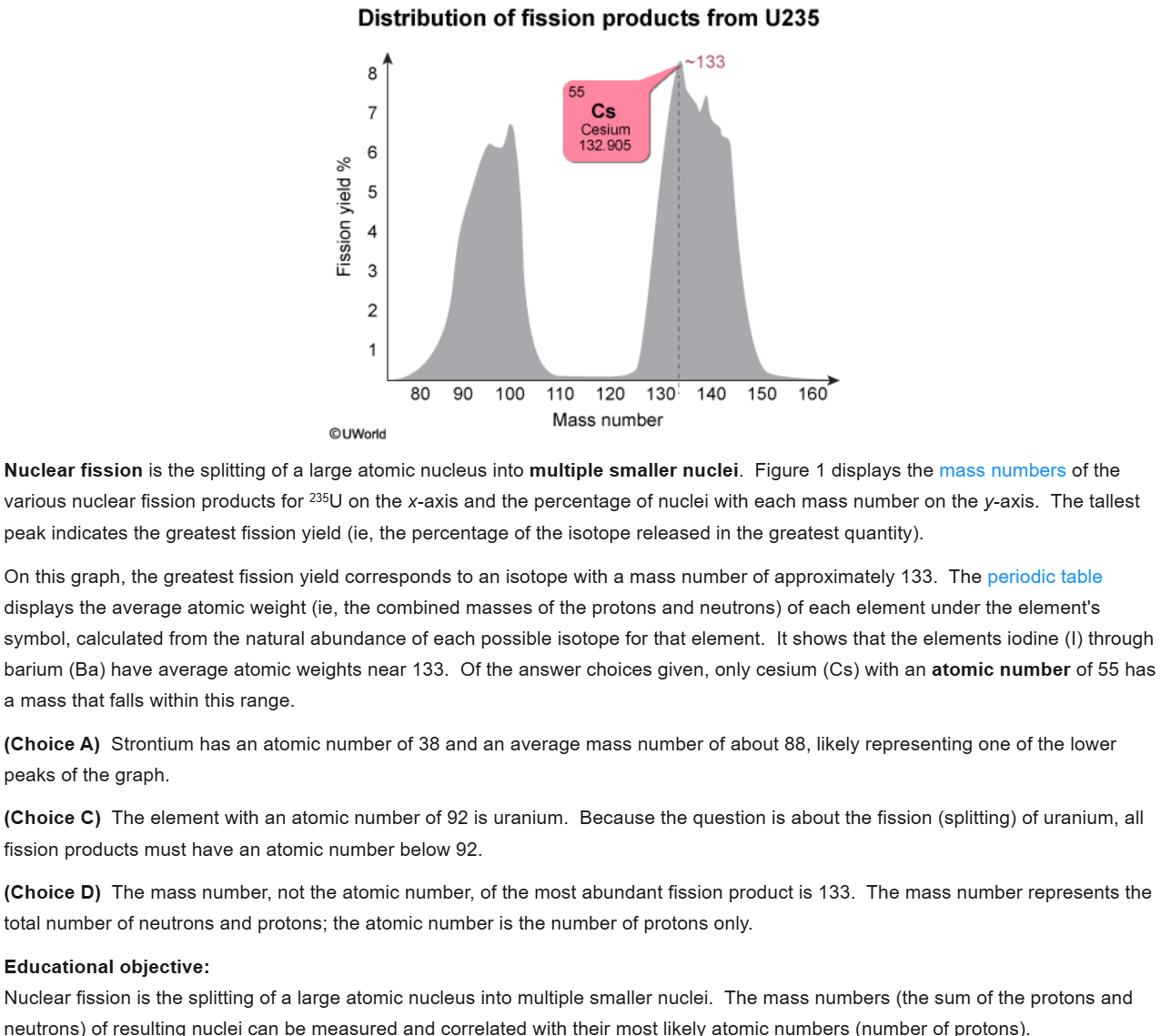
34
New cards
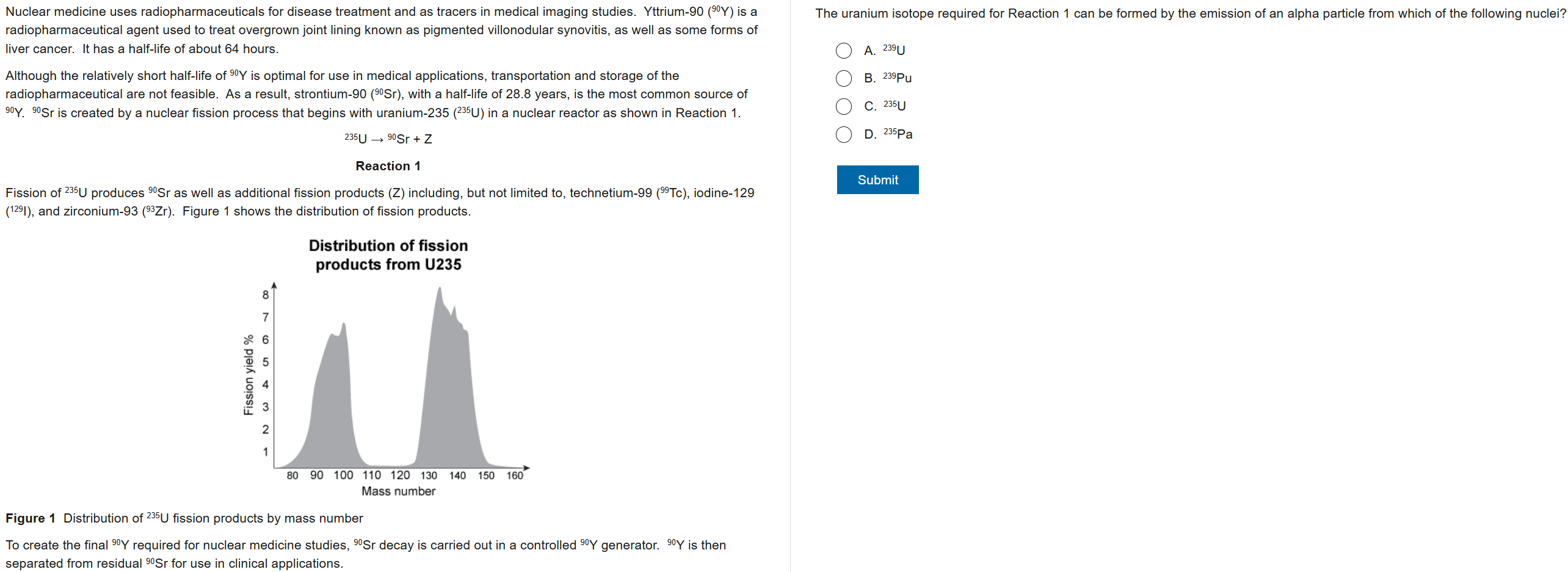
B
35
New cards
C
36
New cards

B
37
New cards

B
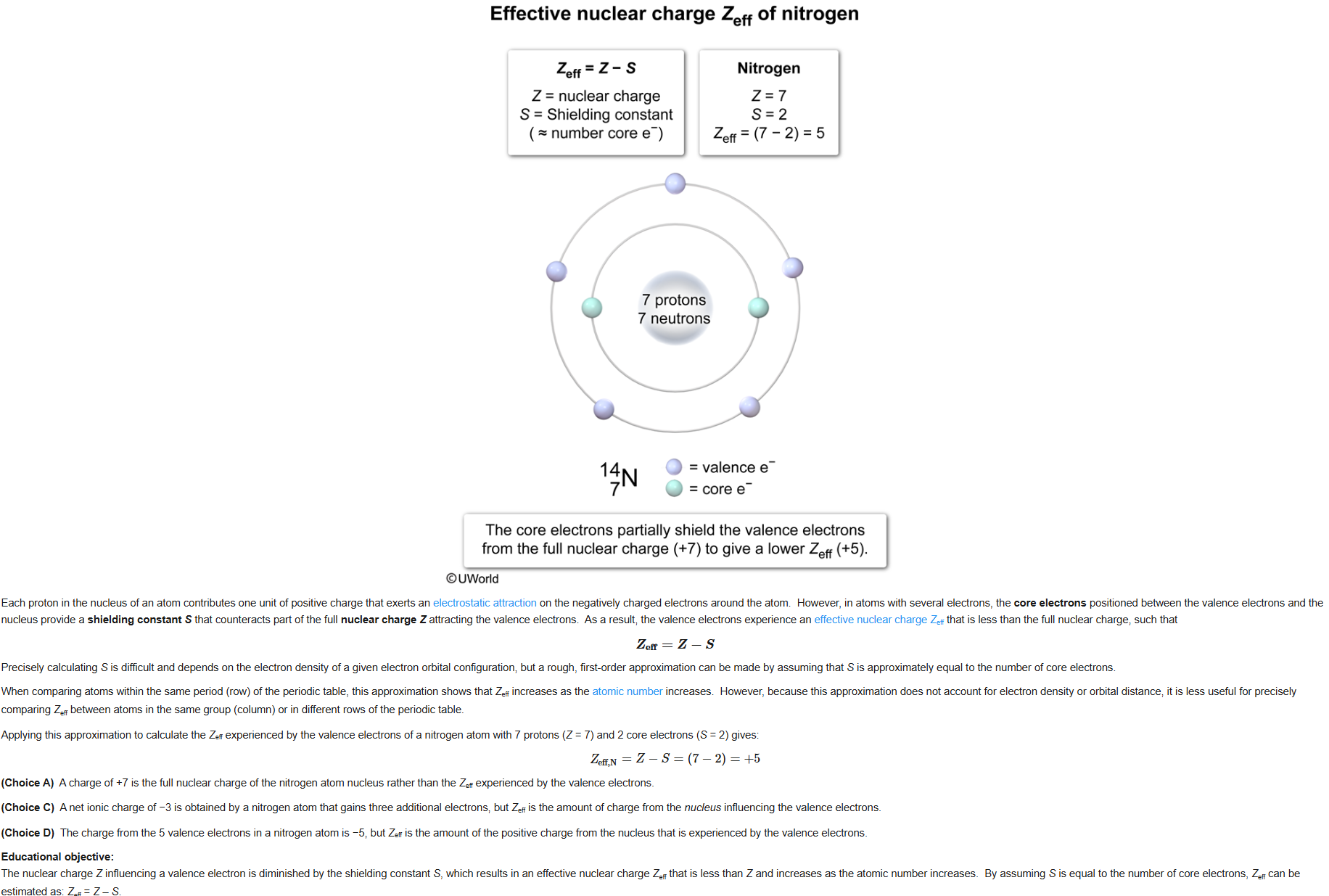
38
New cards
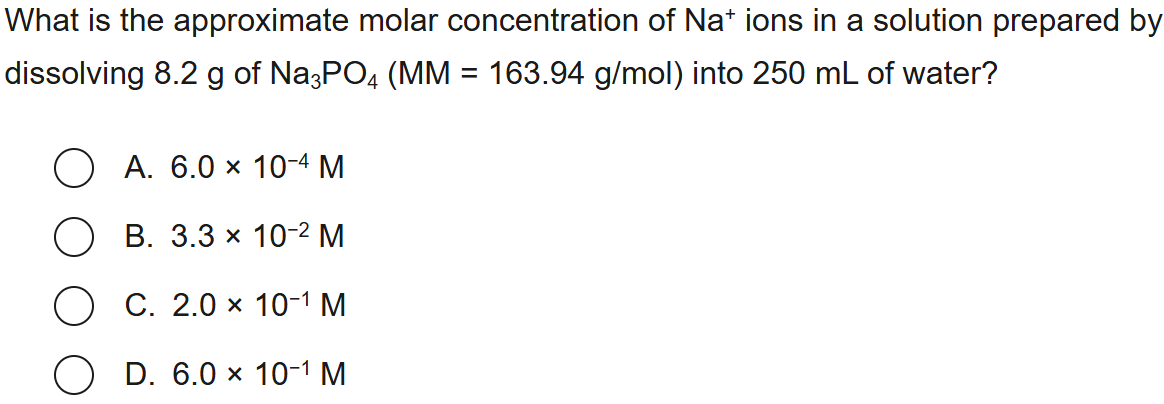
D
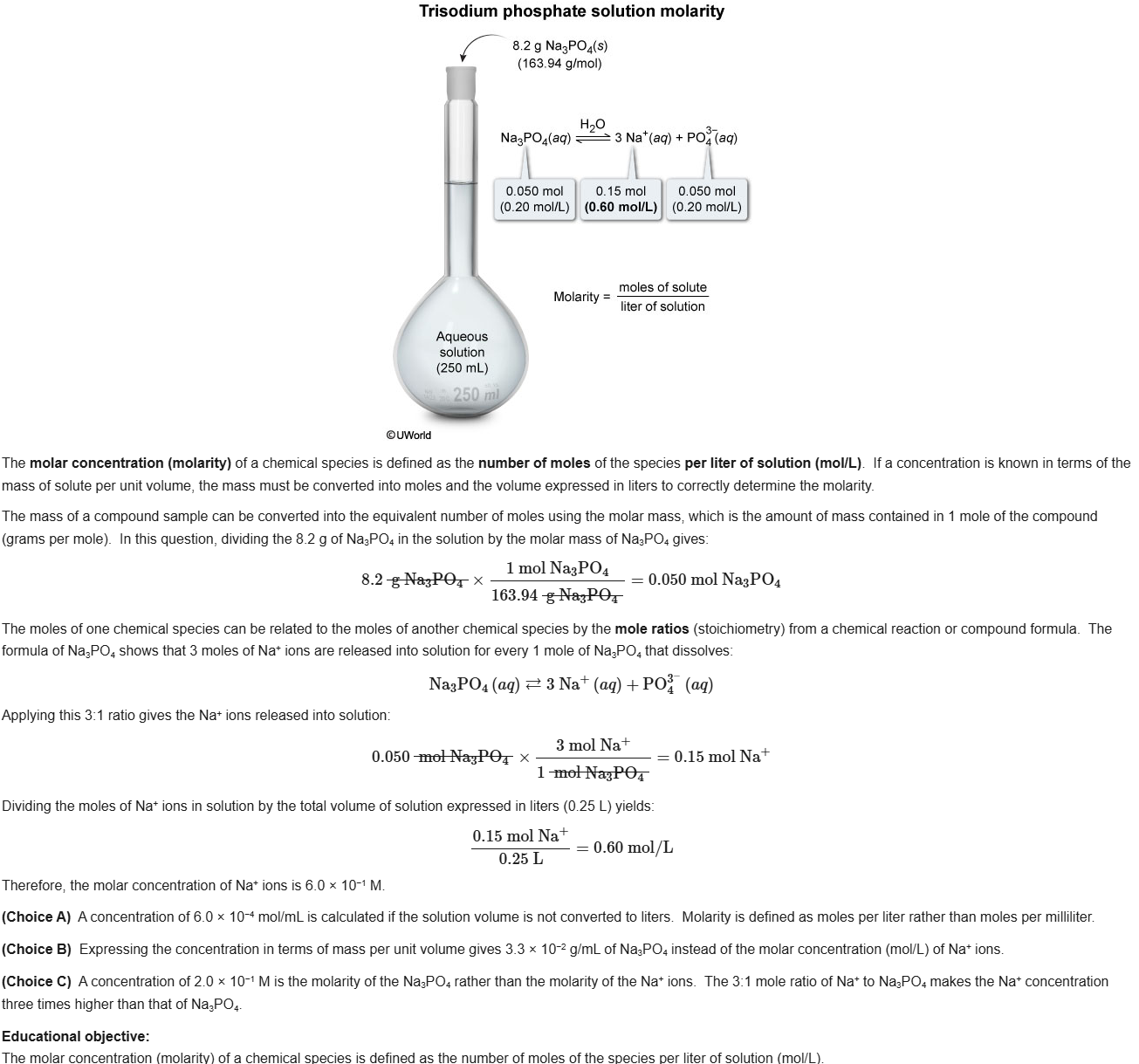
39
New cards
C
40
New cards
B
41
New cards
B
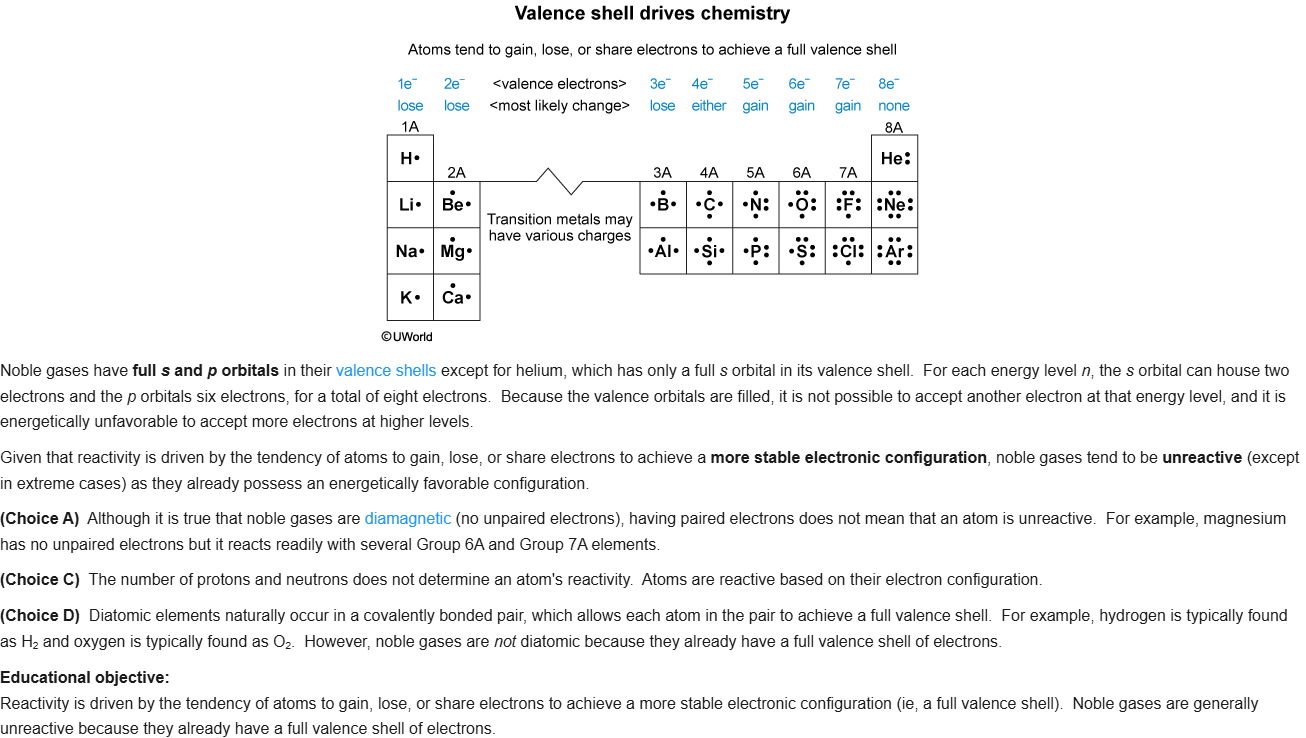
42
New cards
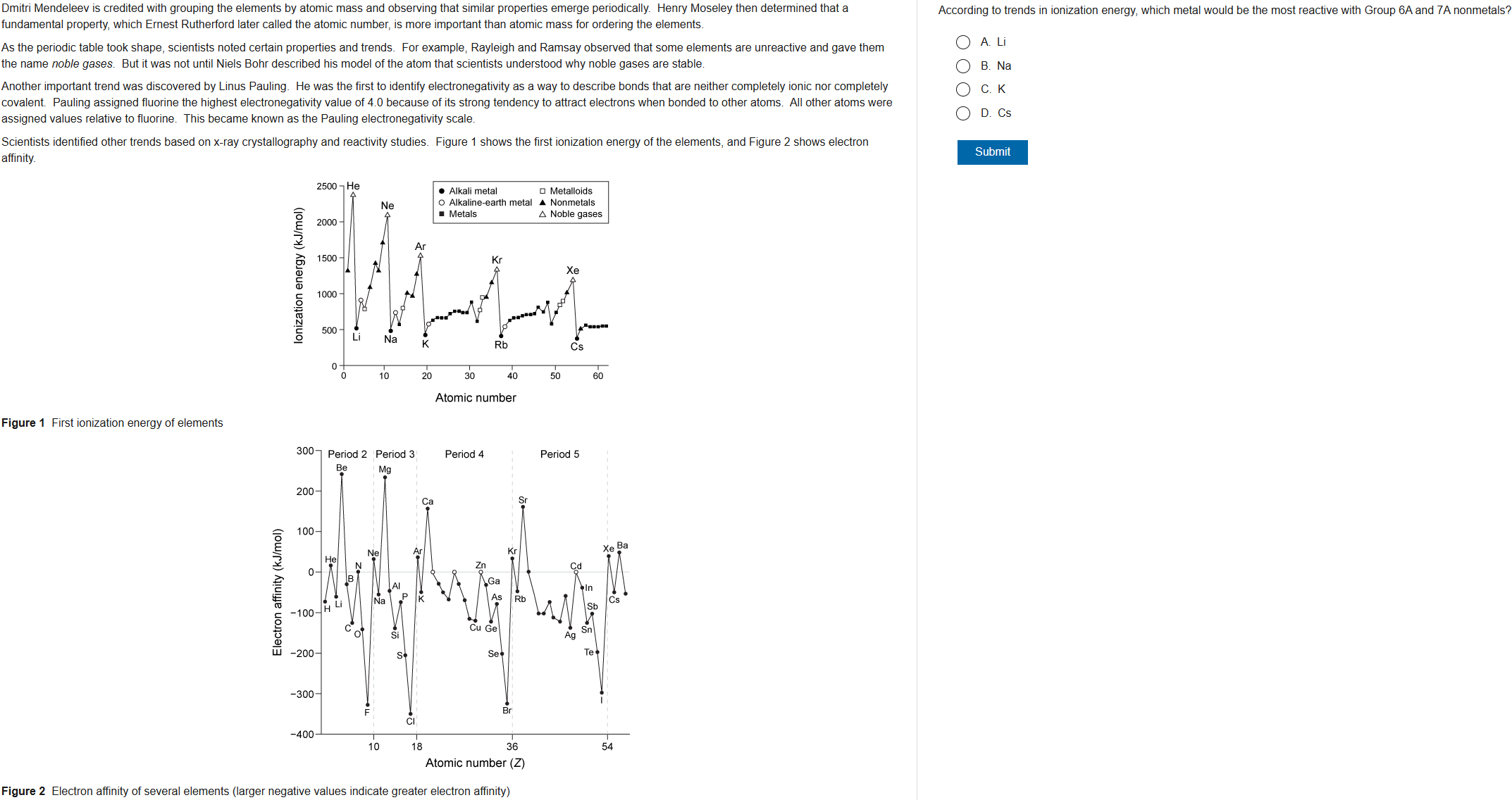
D
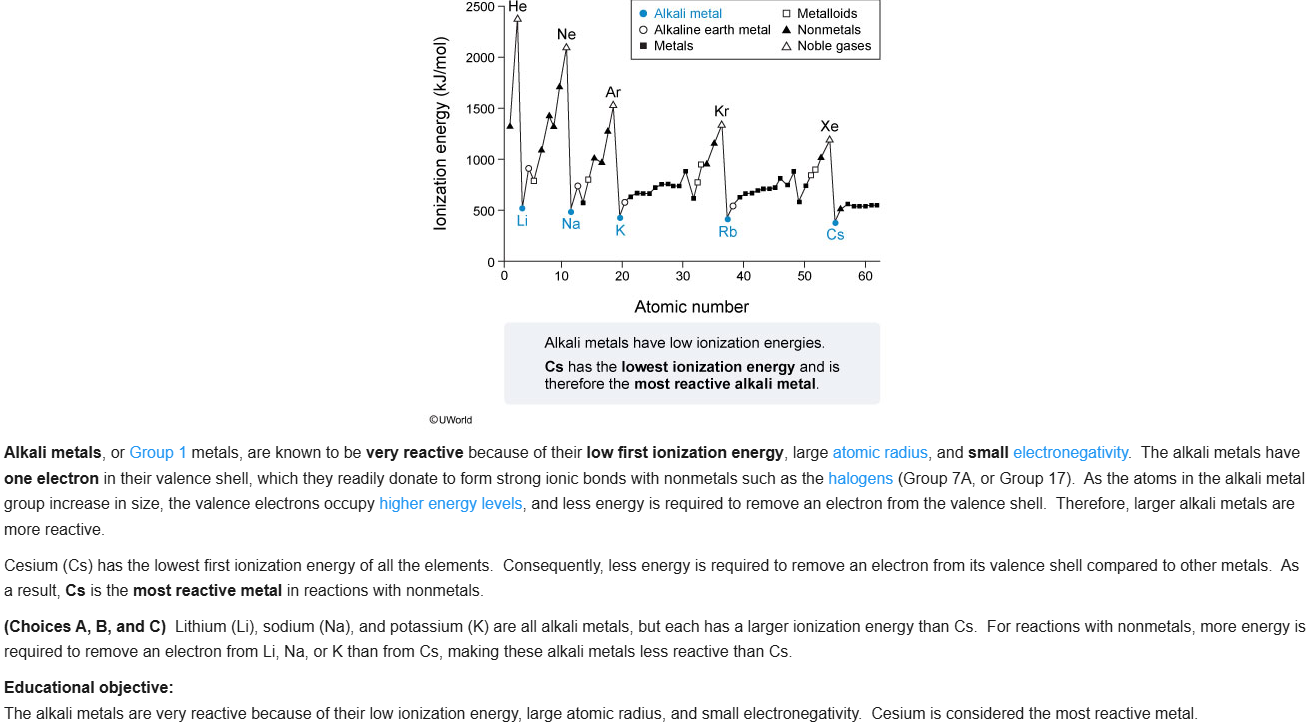
43
New cards
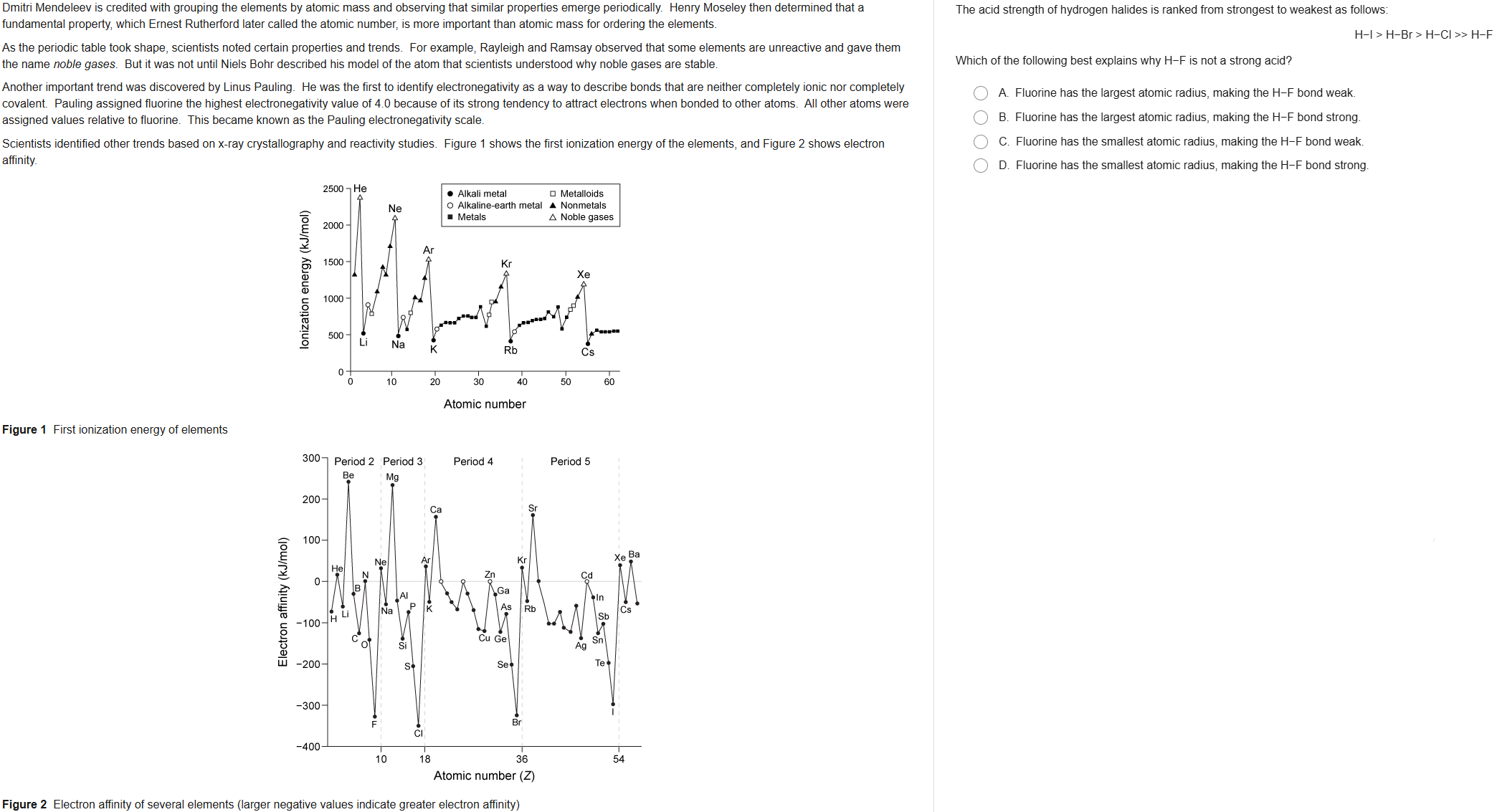
D
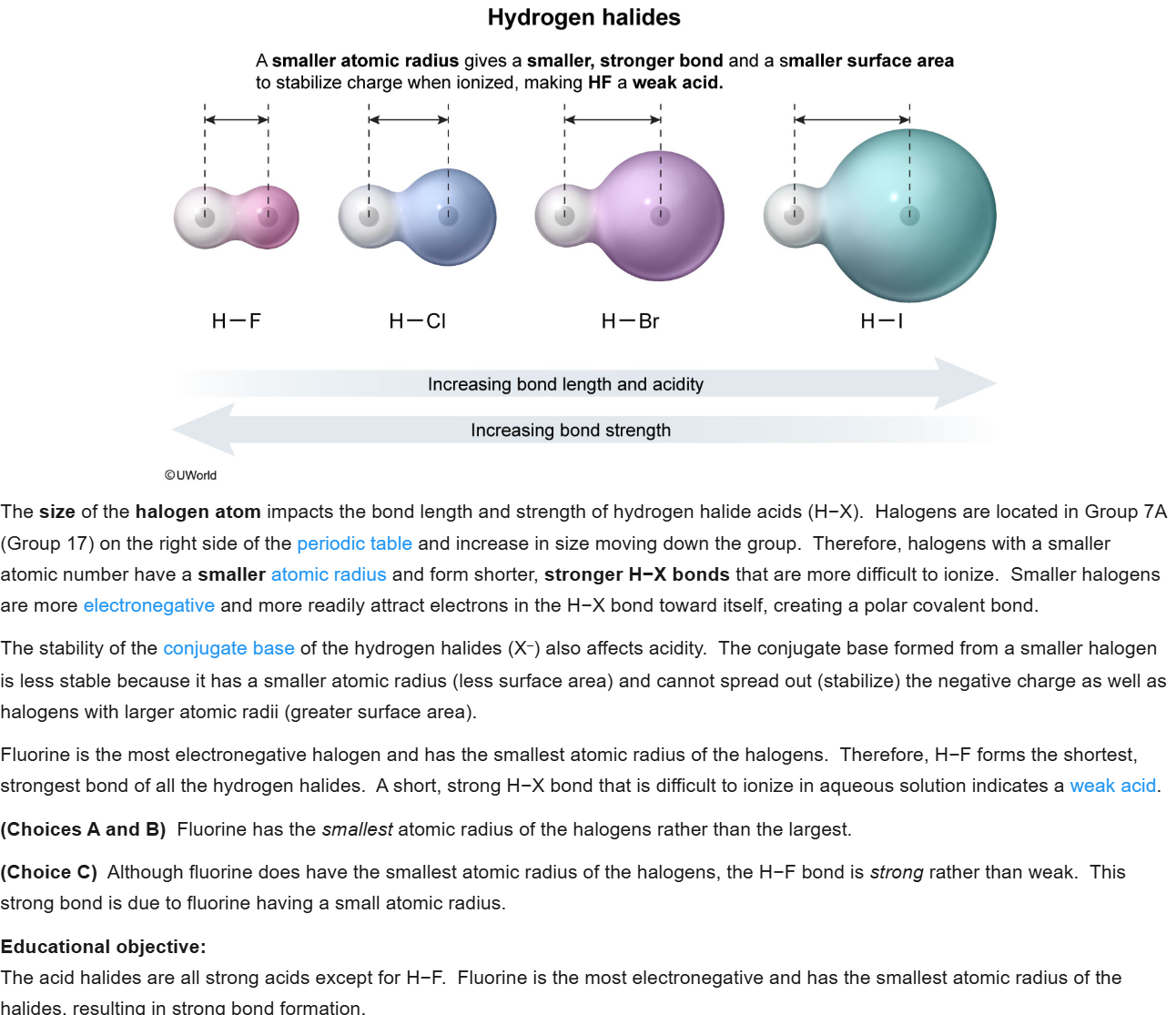
44
New cards
B
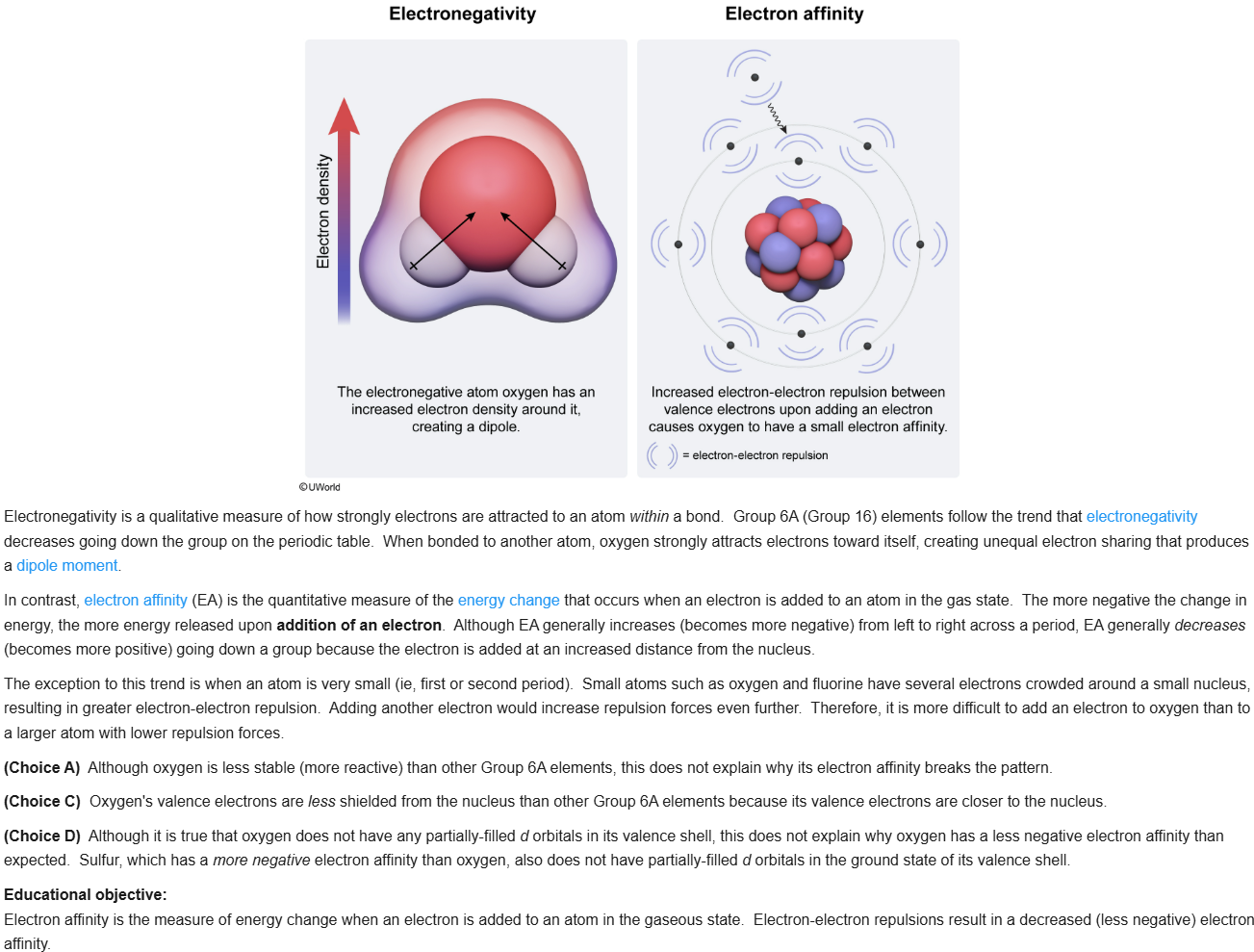
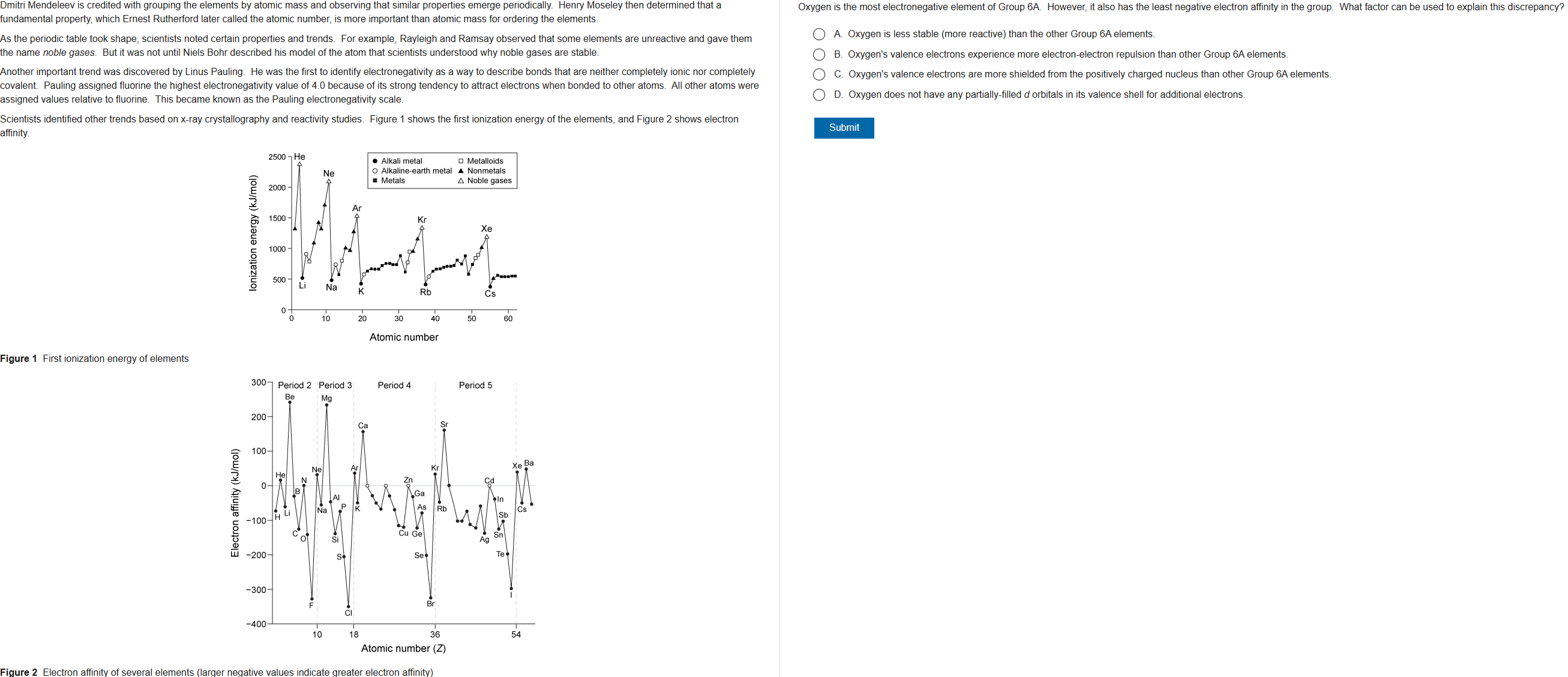
45
New cards
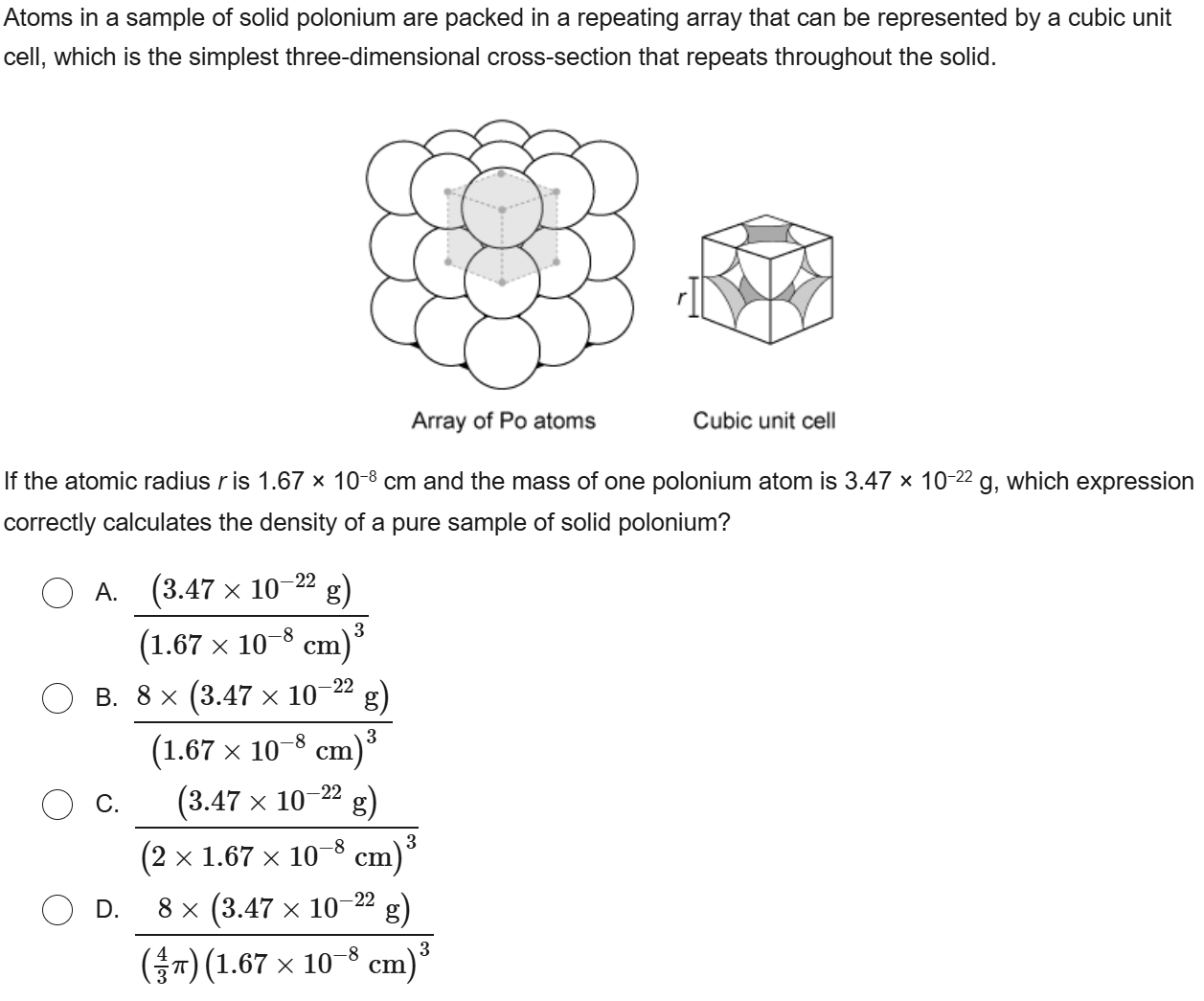
C
46
New cards

A
47
New cards
D
48
New cards
B

49
New cards
A
50
New cards
D
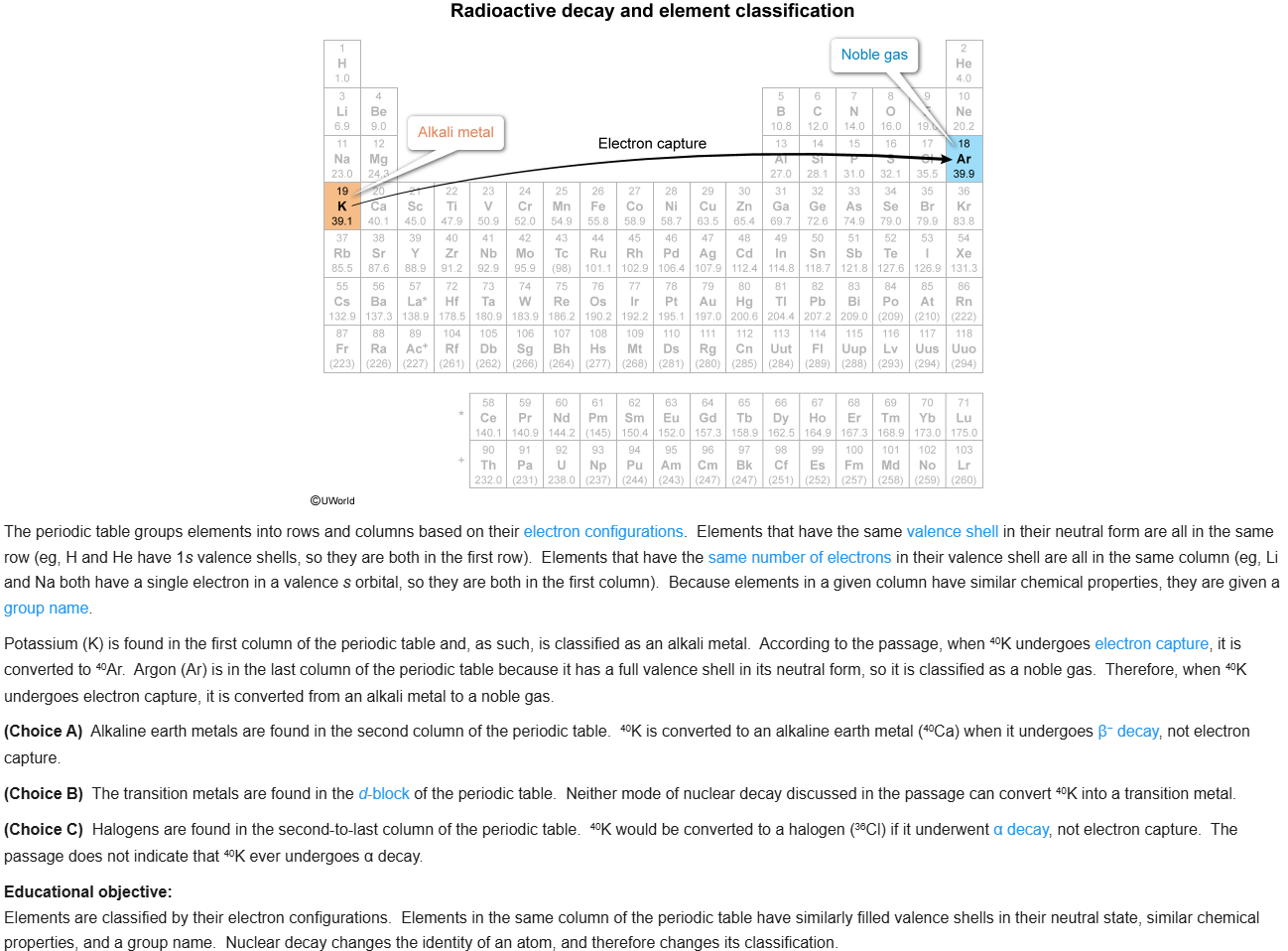
51
New cards

D
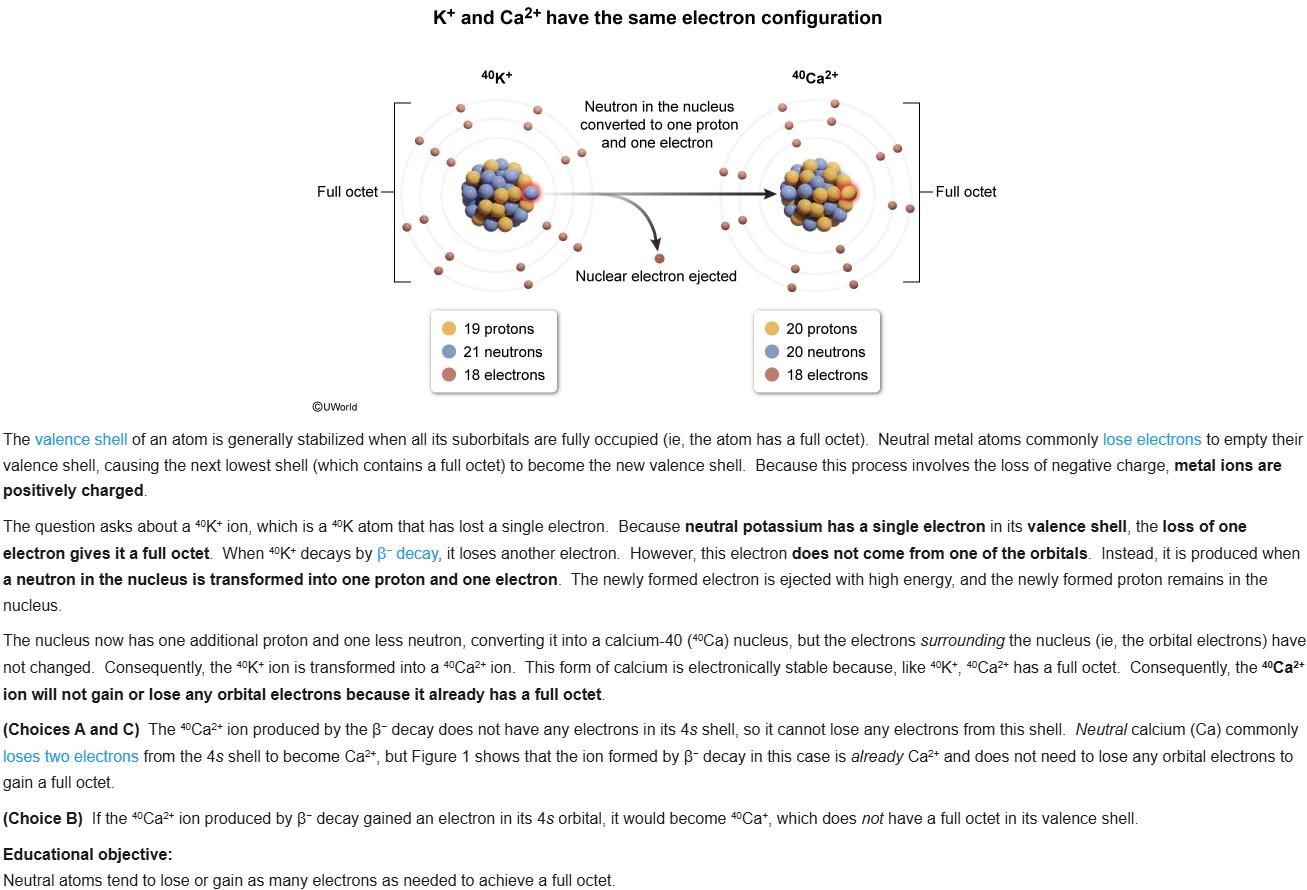
52
New cards
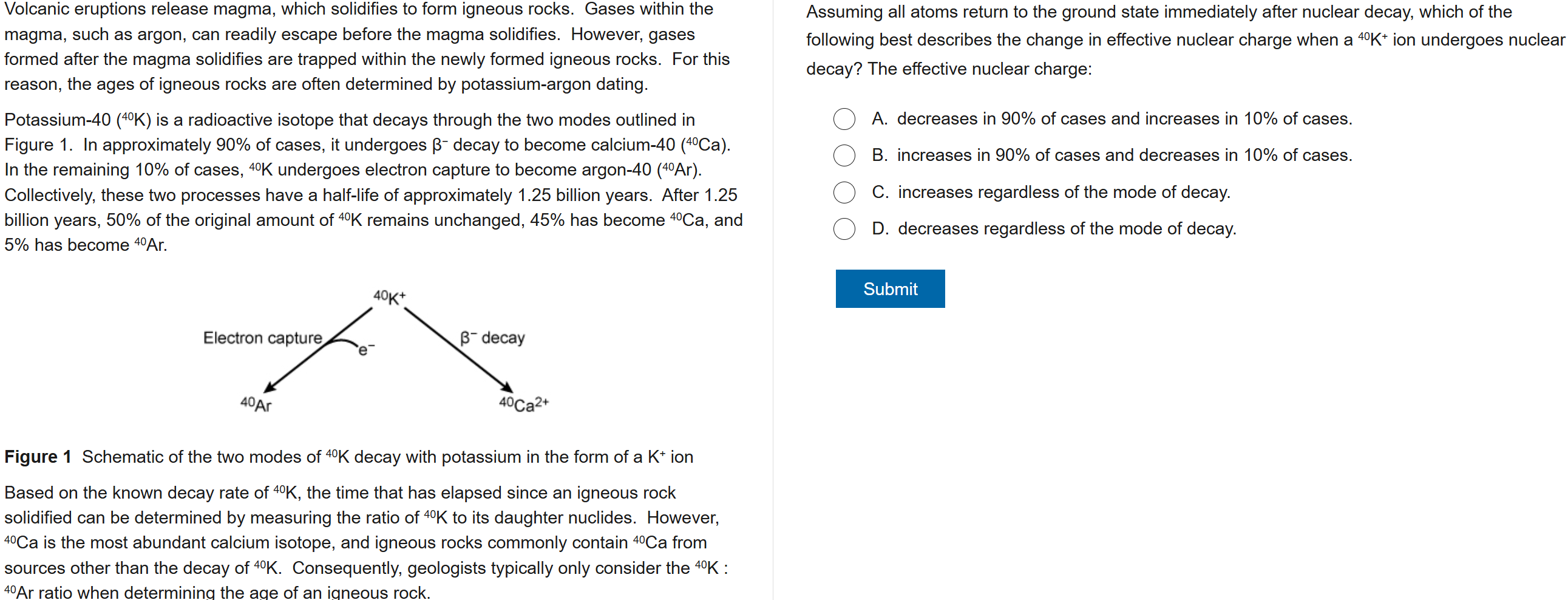
B
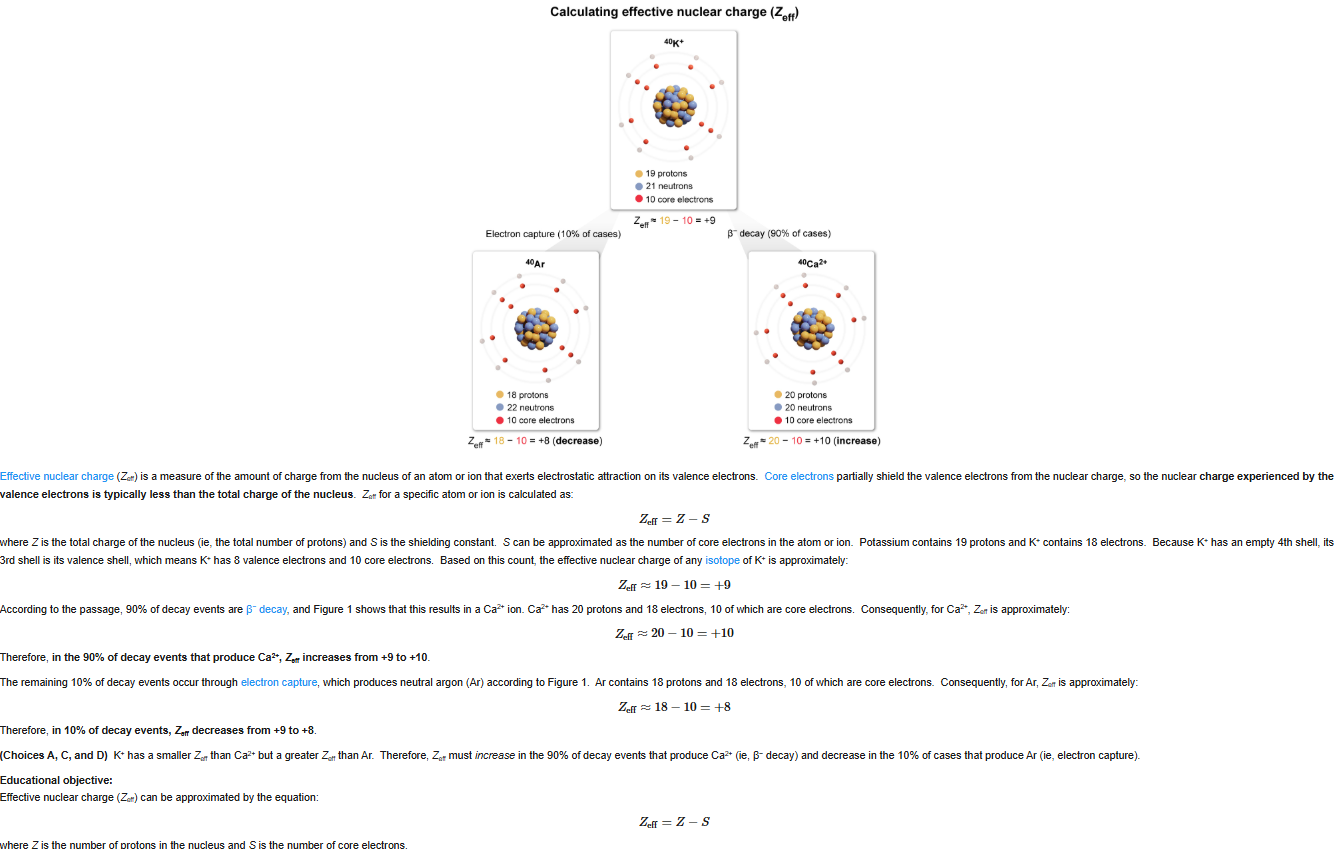
53
New cards
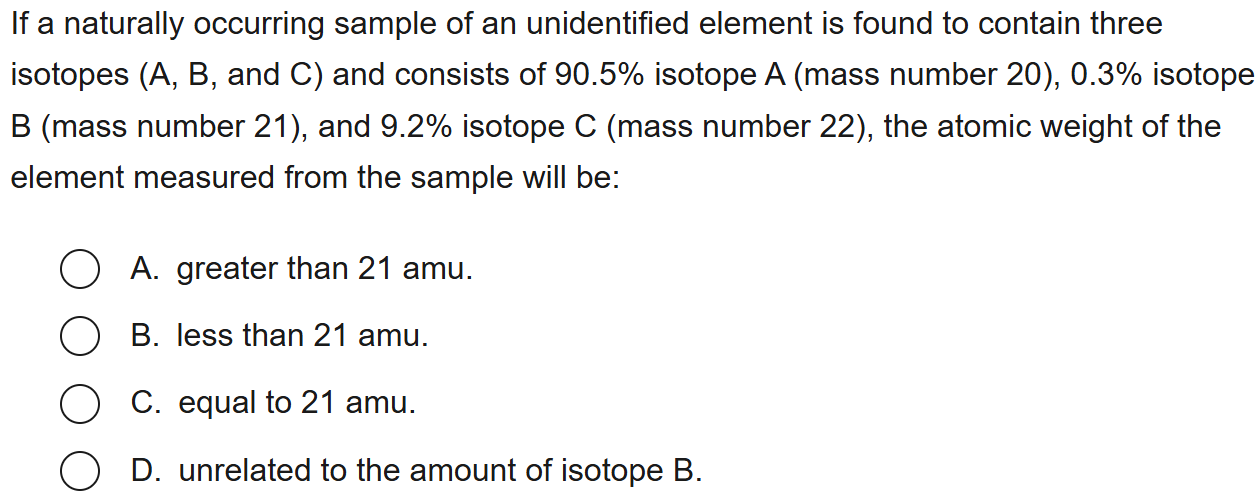
B
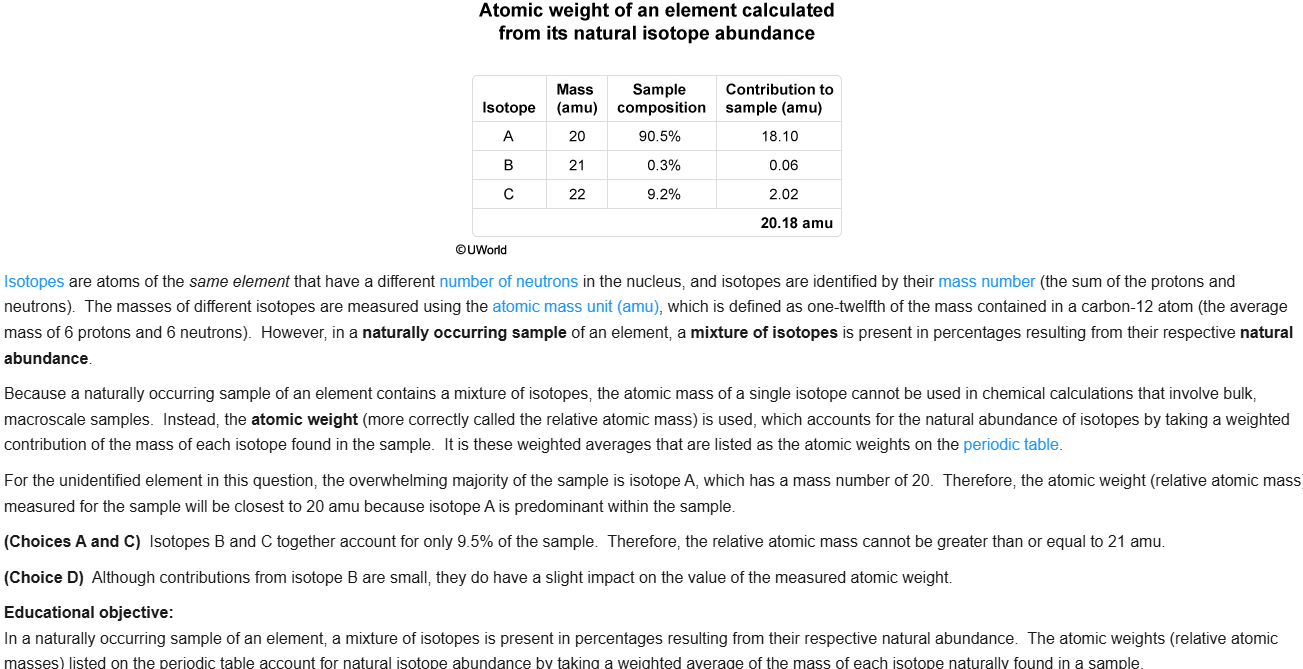
54
New cards
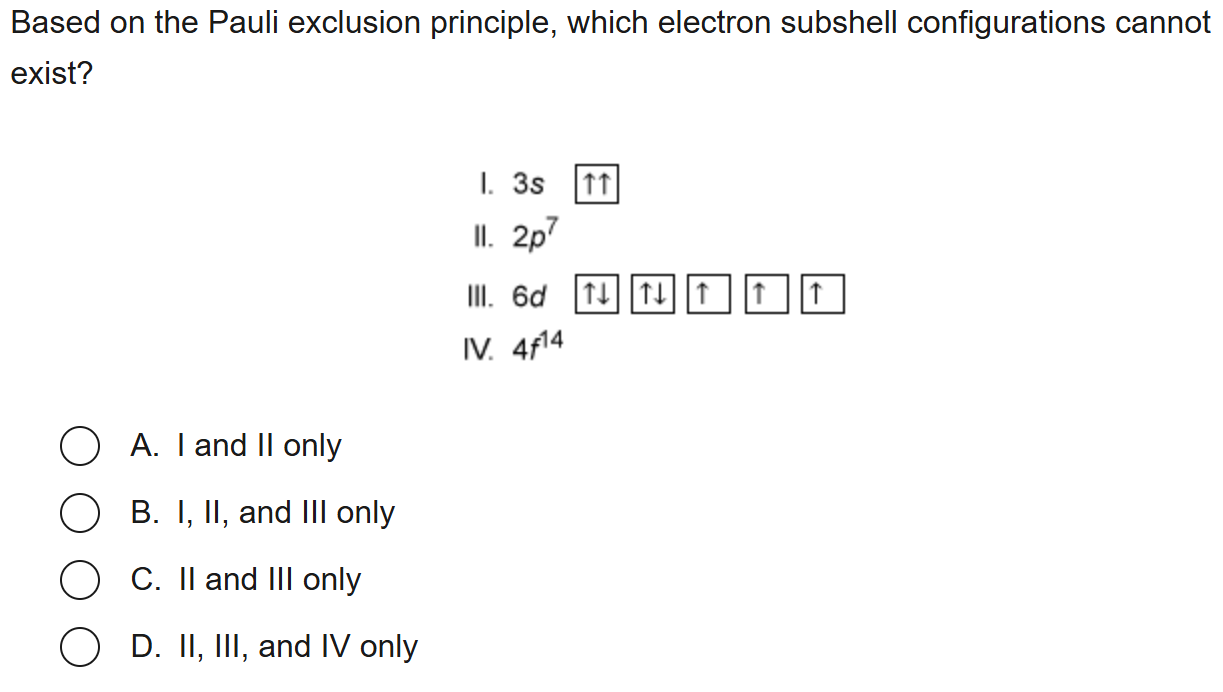
A
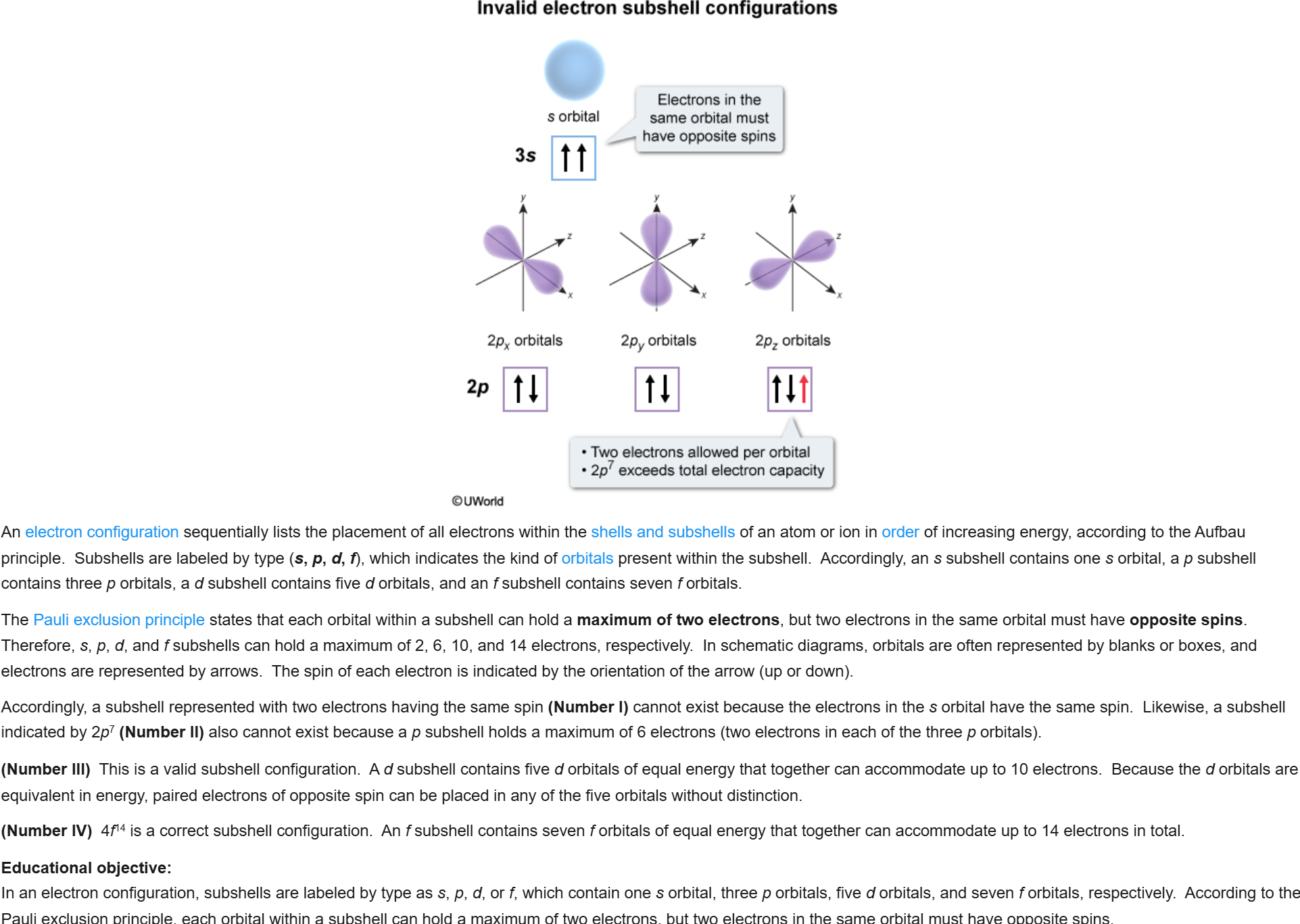
55
New cards

D
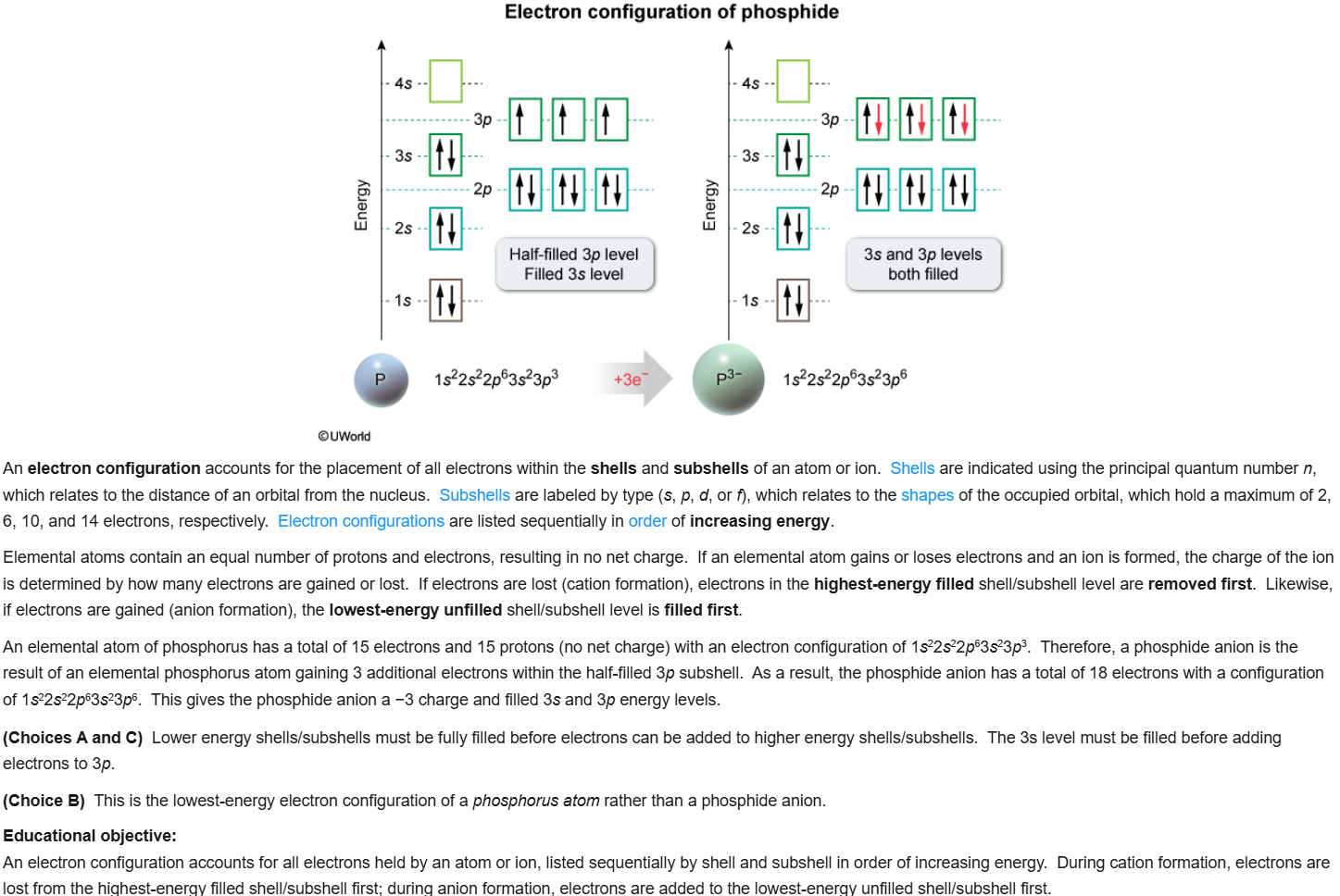
56
New cards
C

57
New cards
D
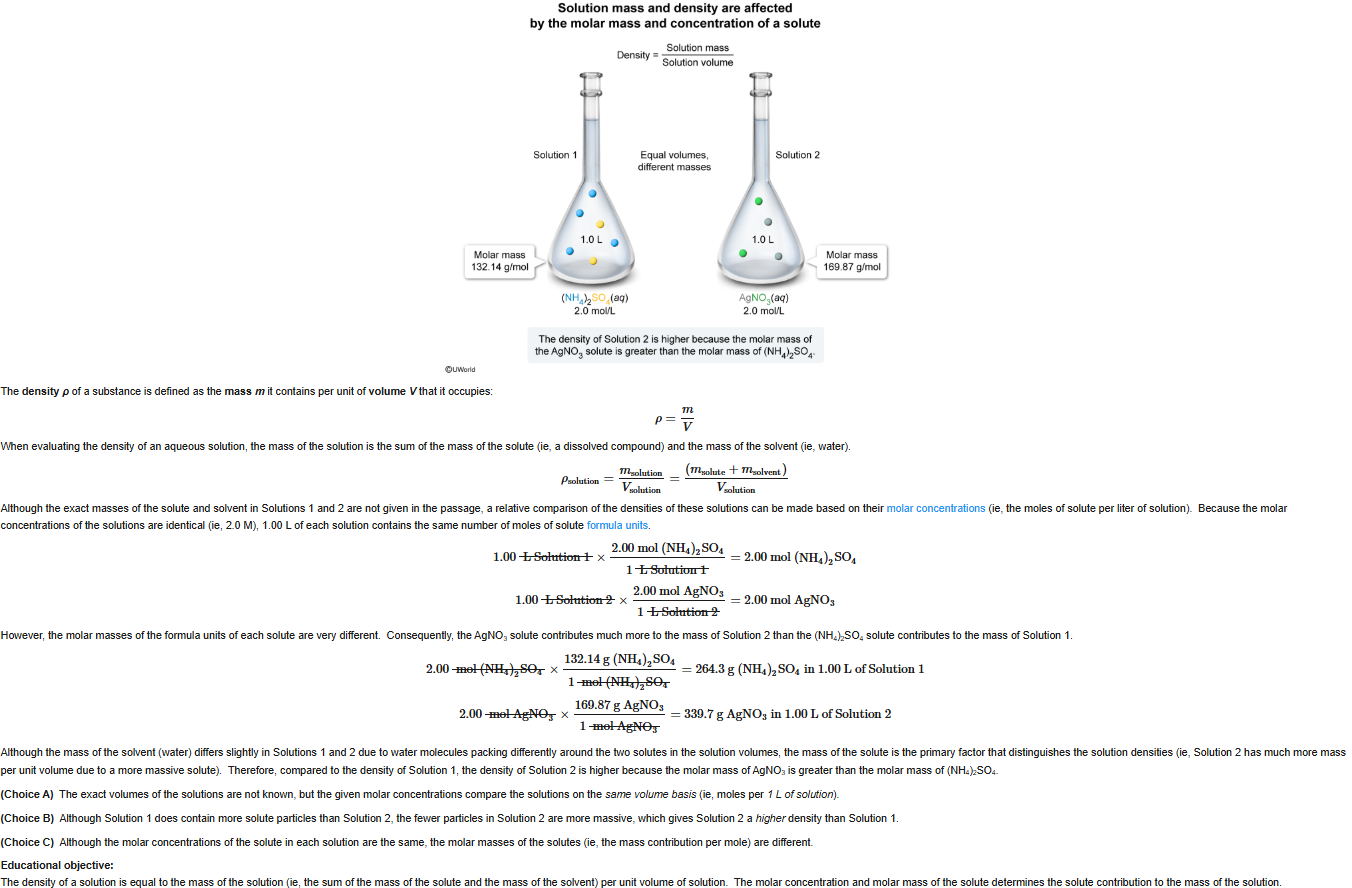
58
New cards
A

59
New cards
C

60
New cards

D

61
New cards

B
62
New cards
B
63
New cards
D

64
New cards
B
65
New cards
A
66
New cards
B
67
New cards
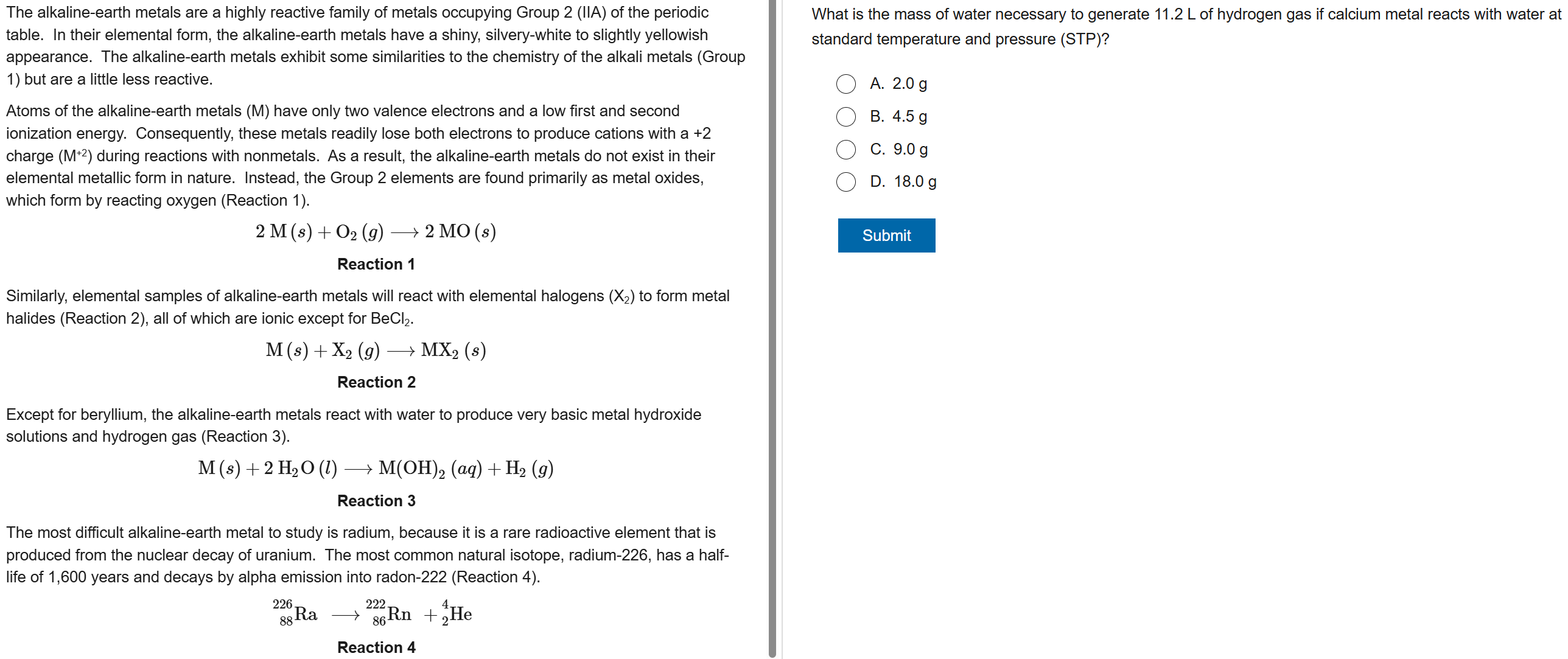
D
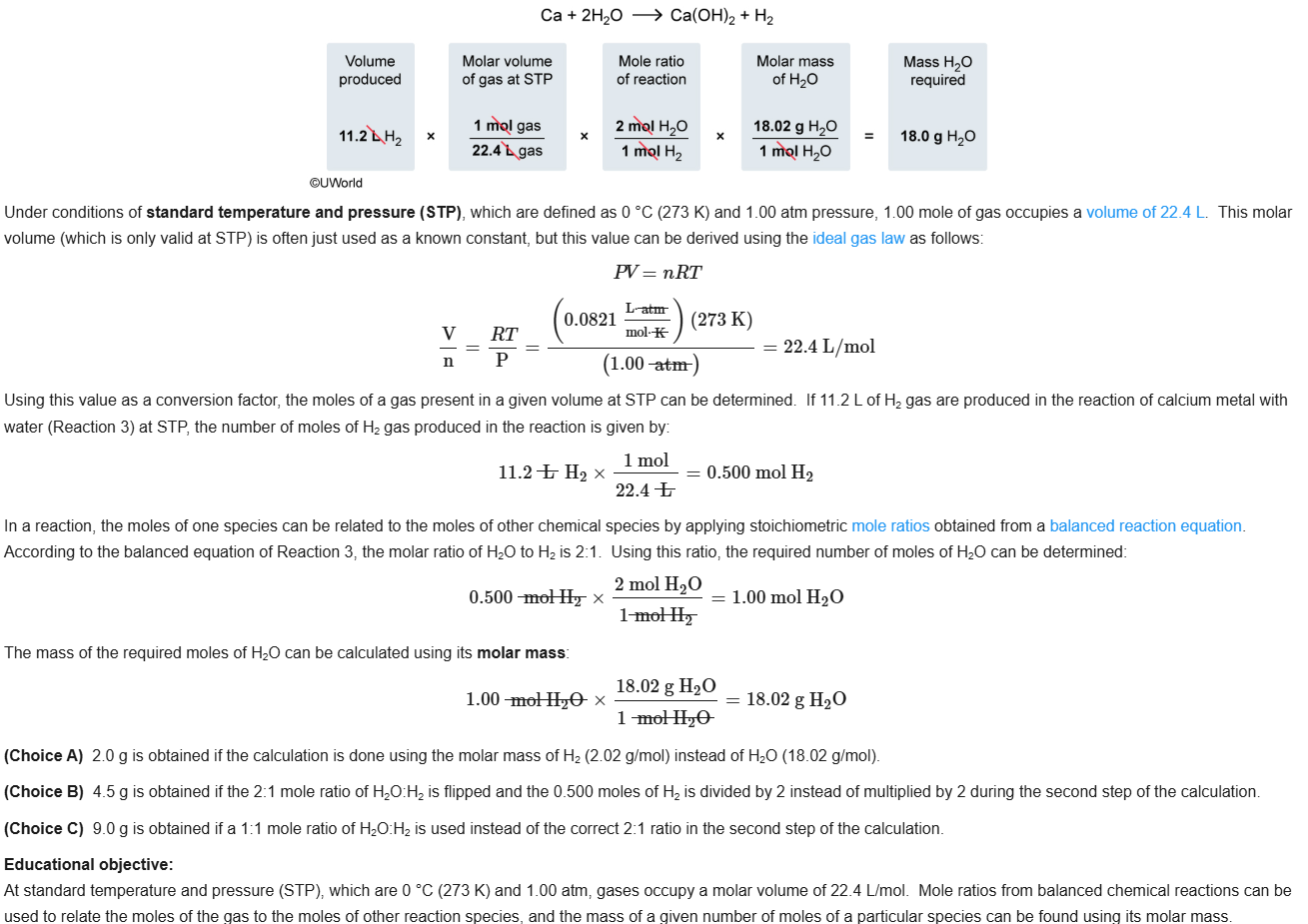
68
New cards
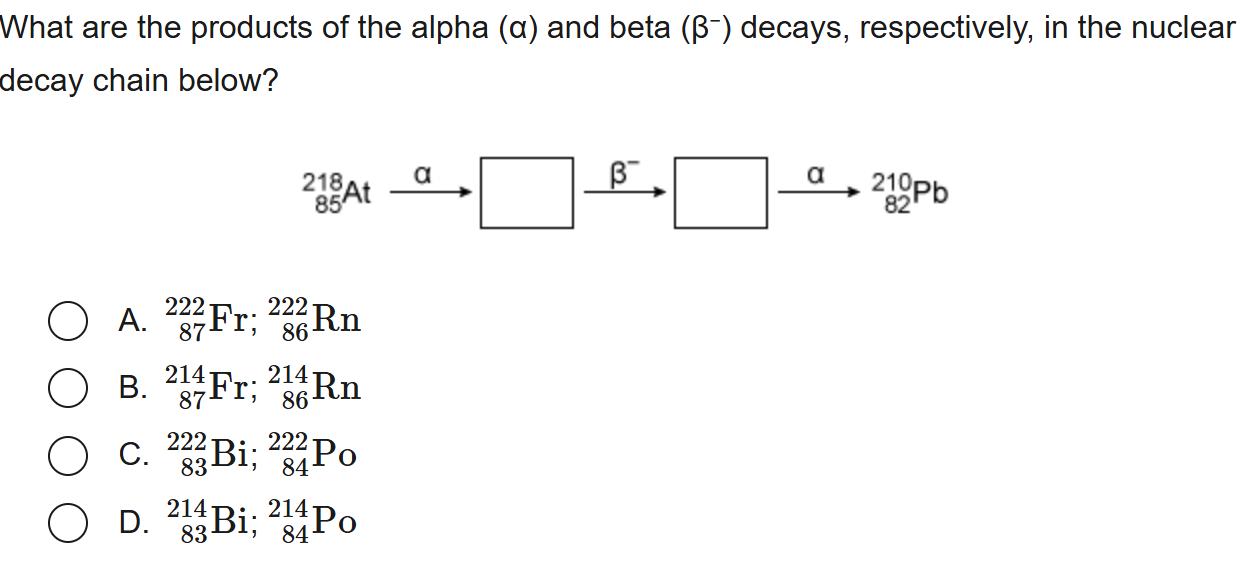
D

69
New cards

C
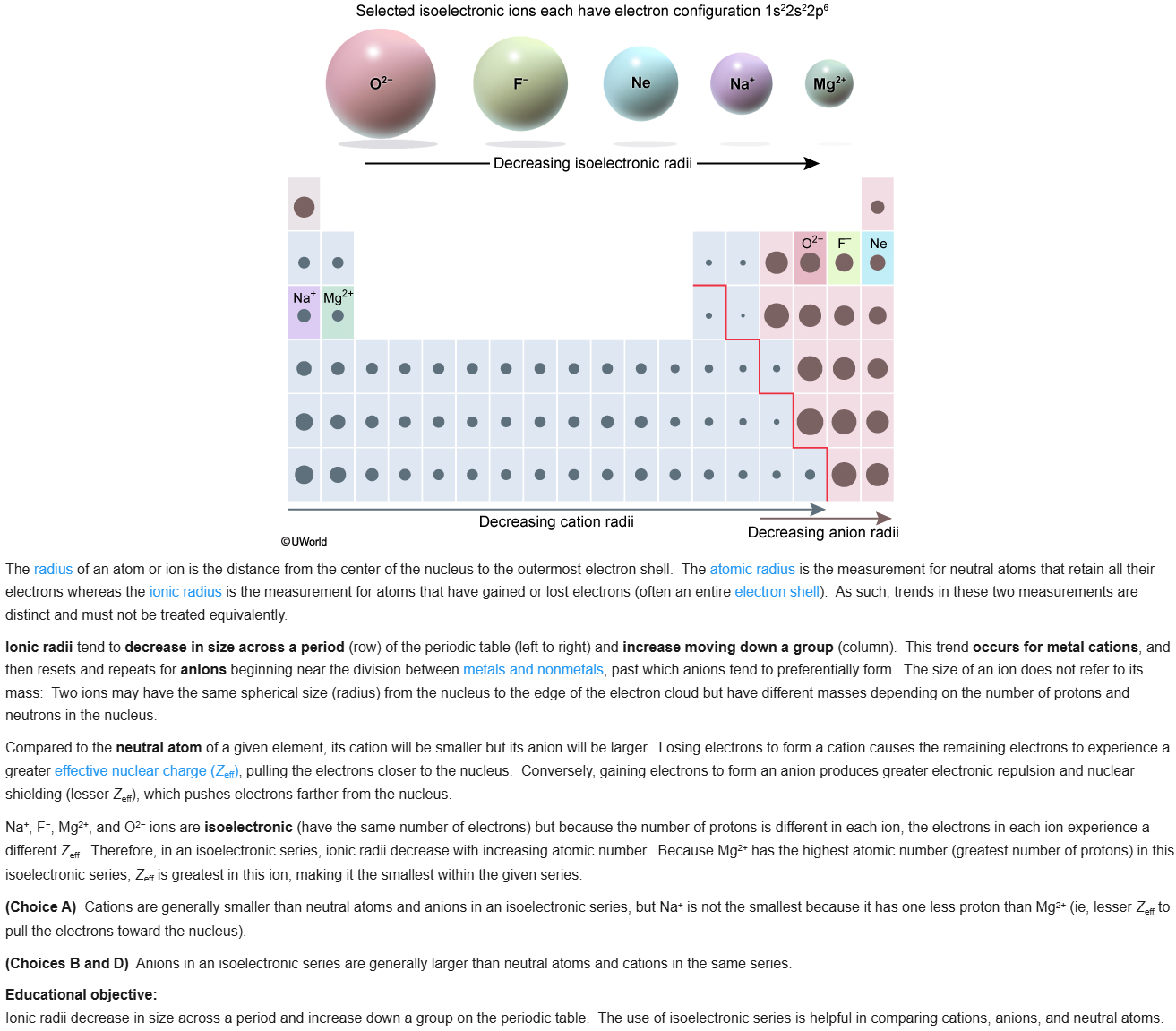
70
New cards
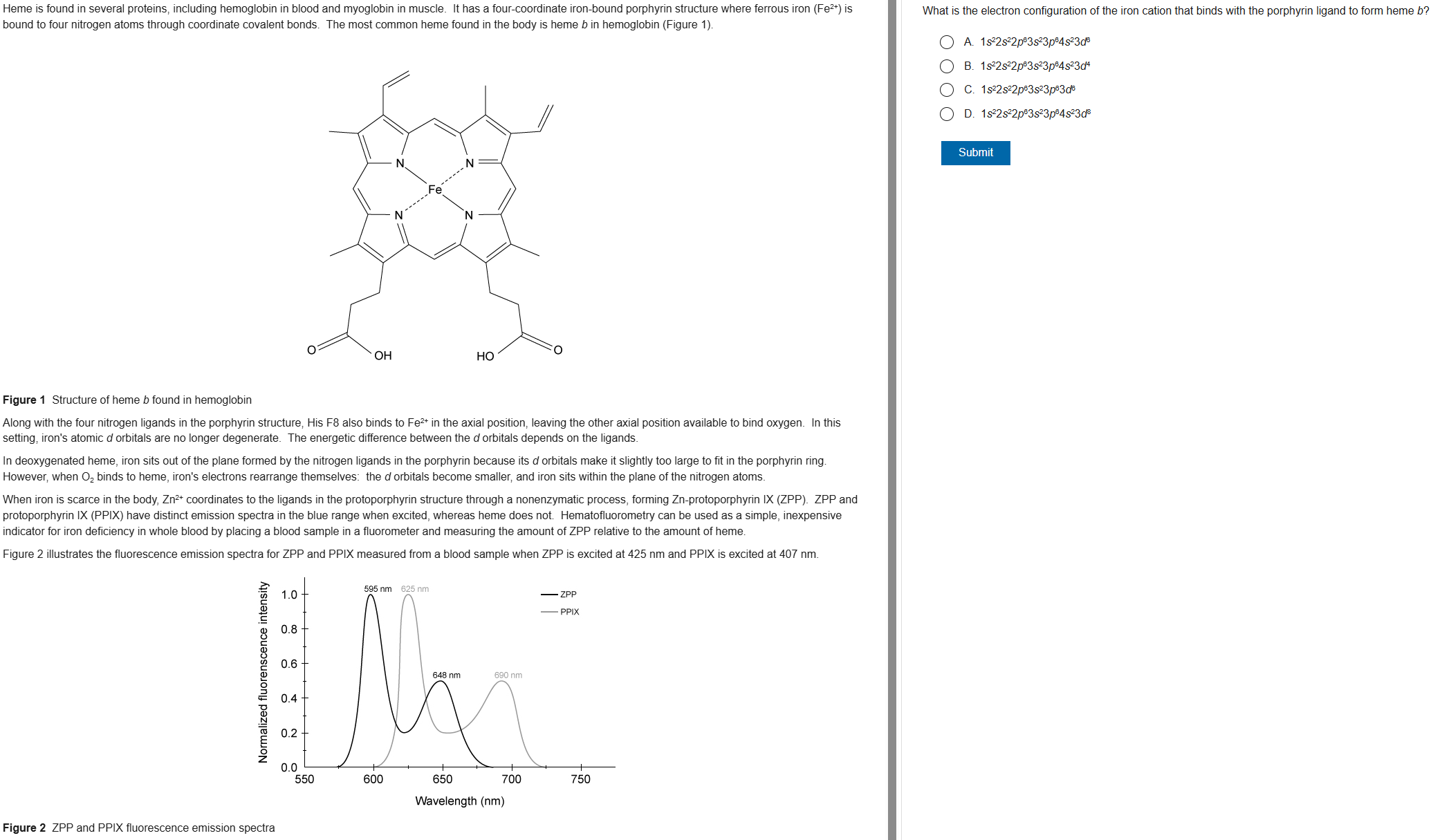
C
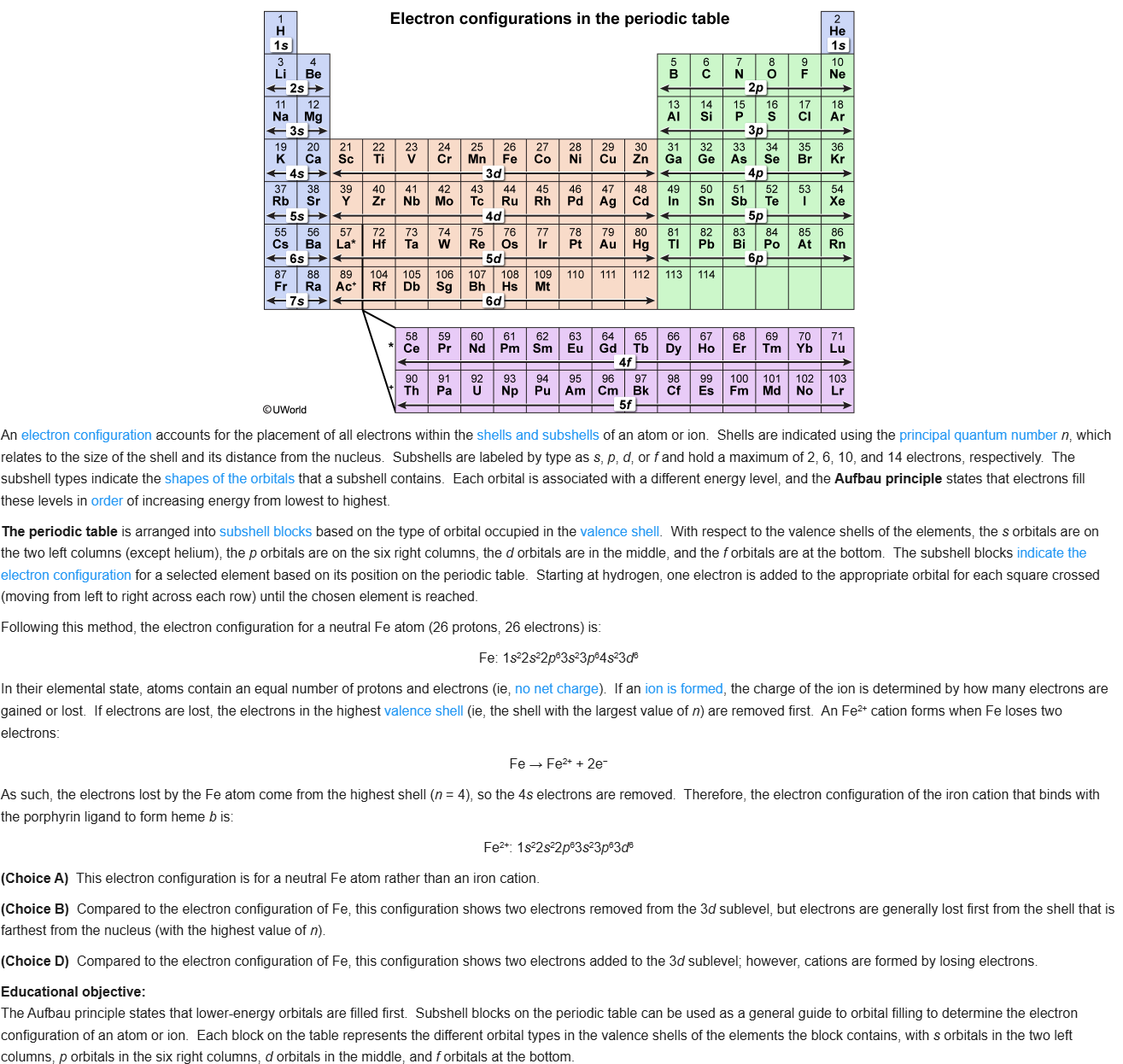
71
New cards

A
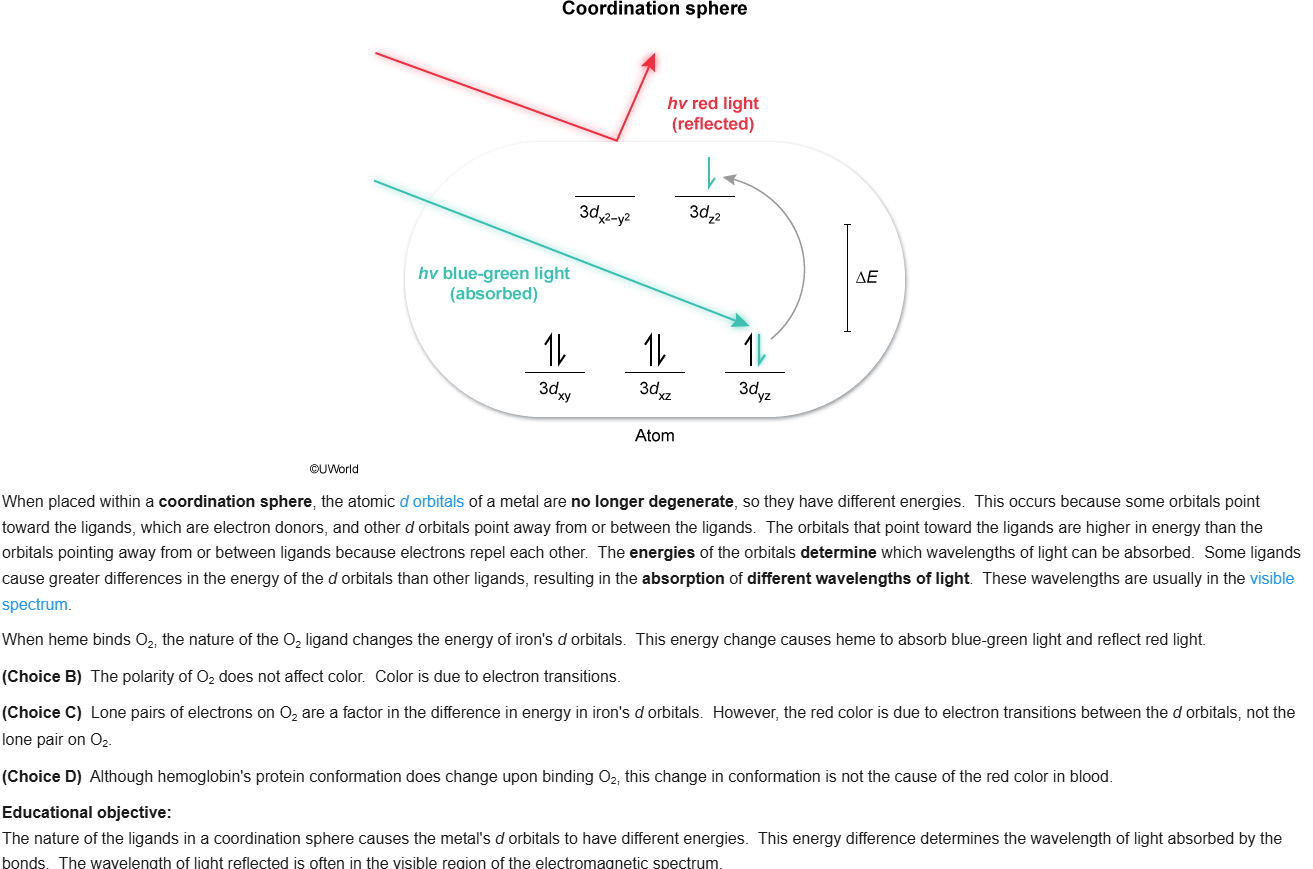
72
New cards
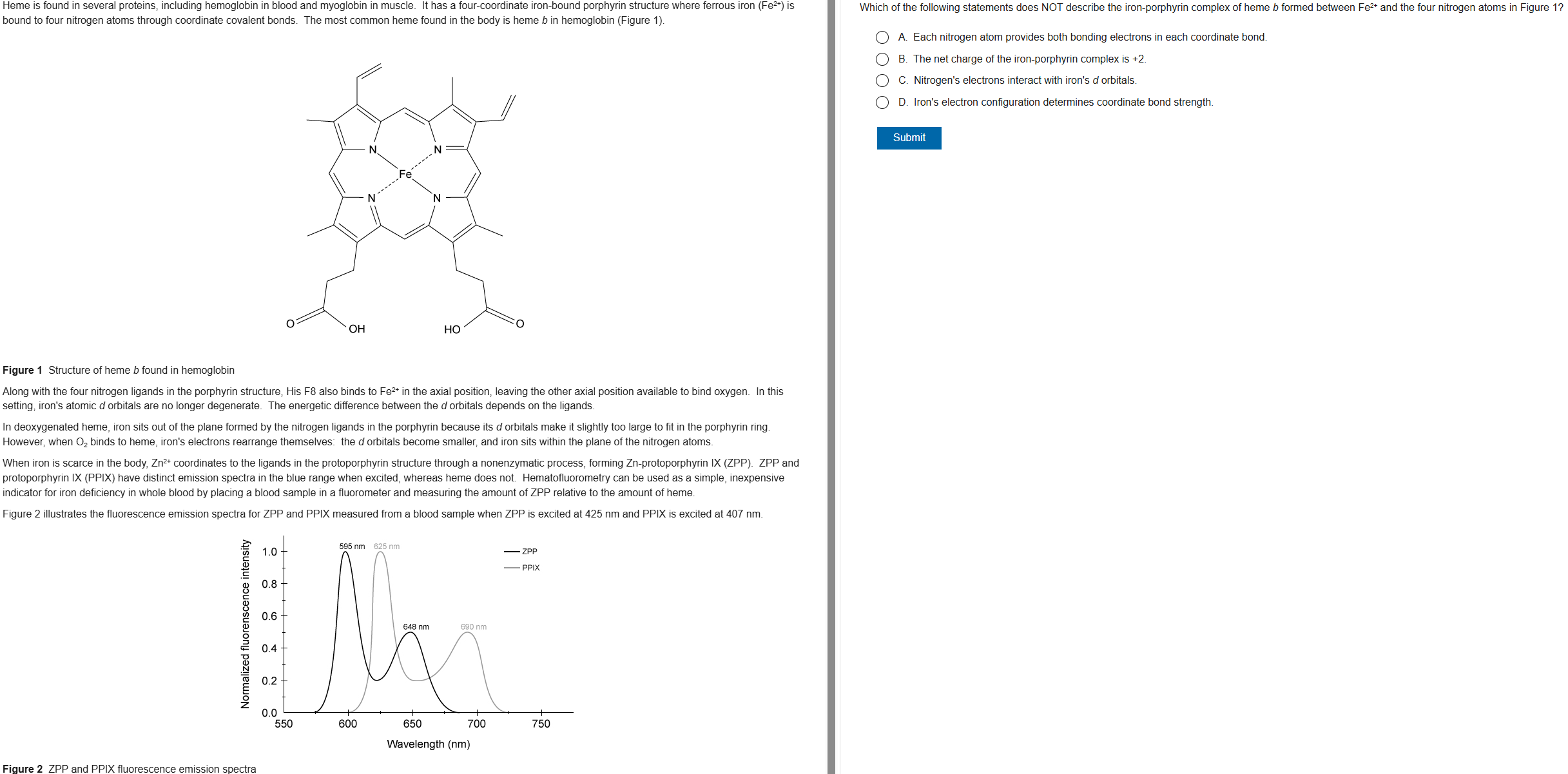
B
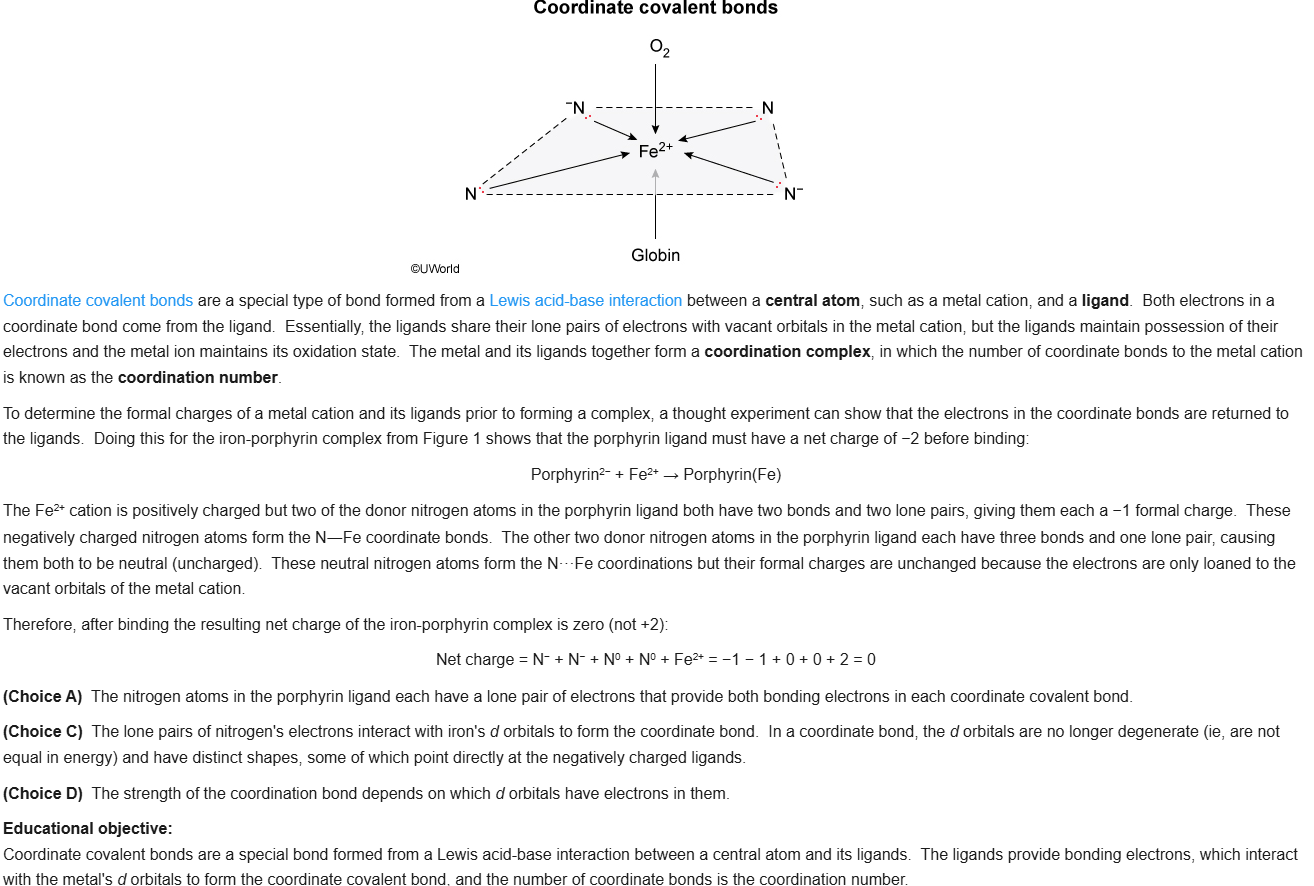
73
New cards
B
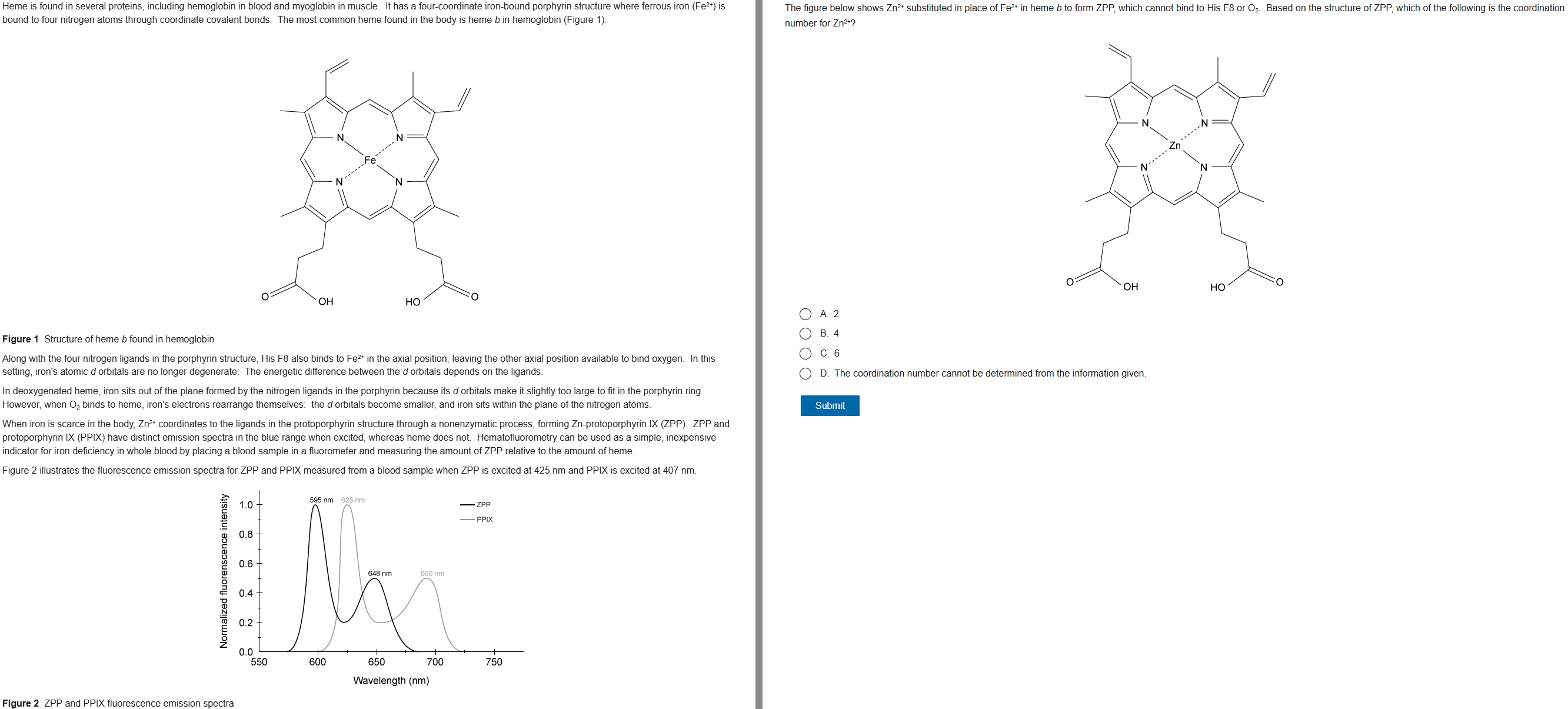
74
New cards

D
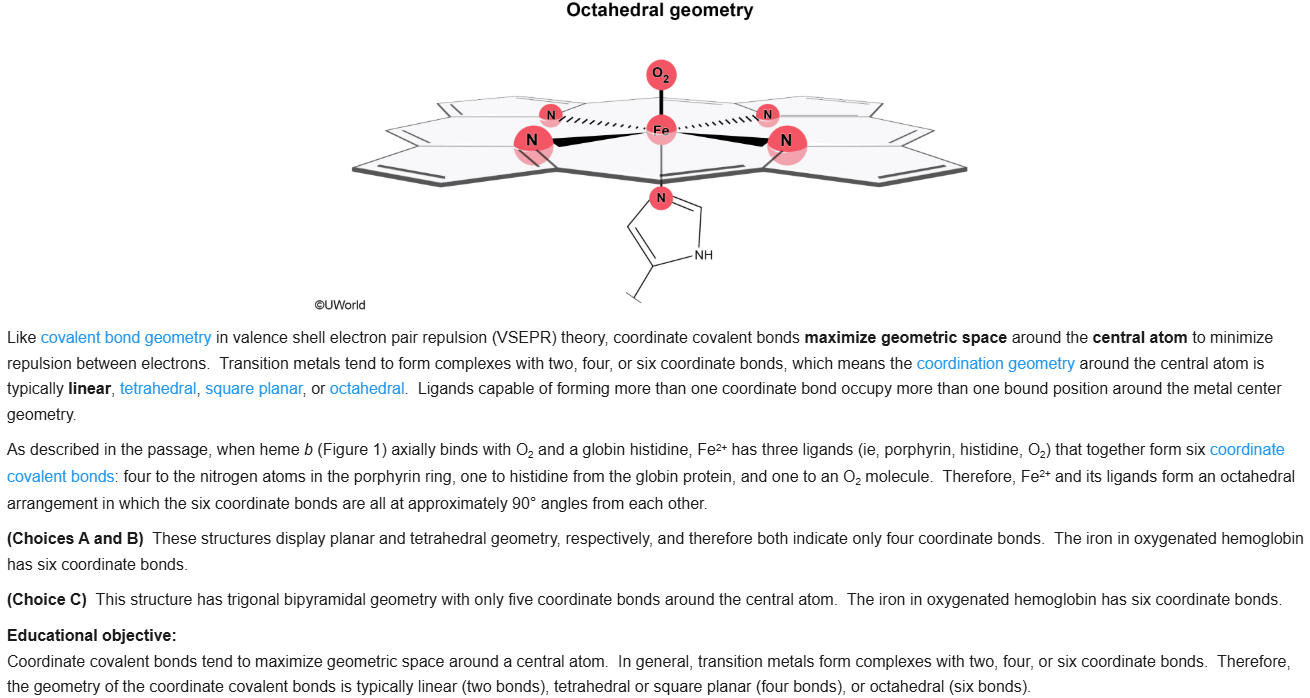
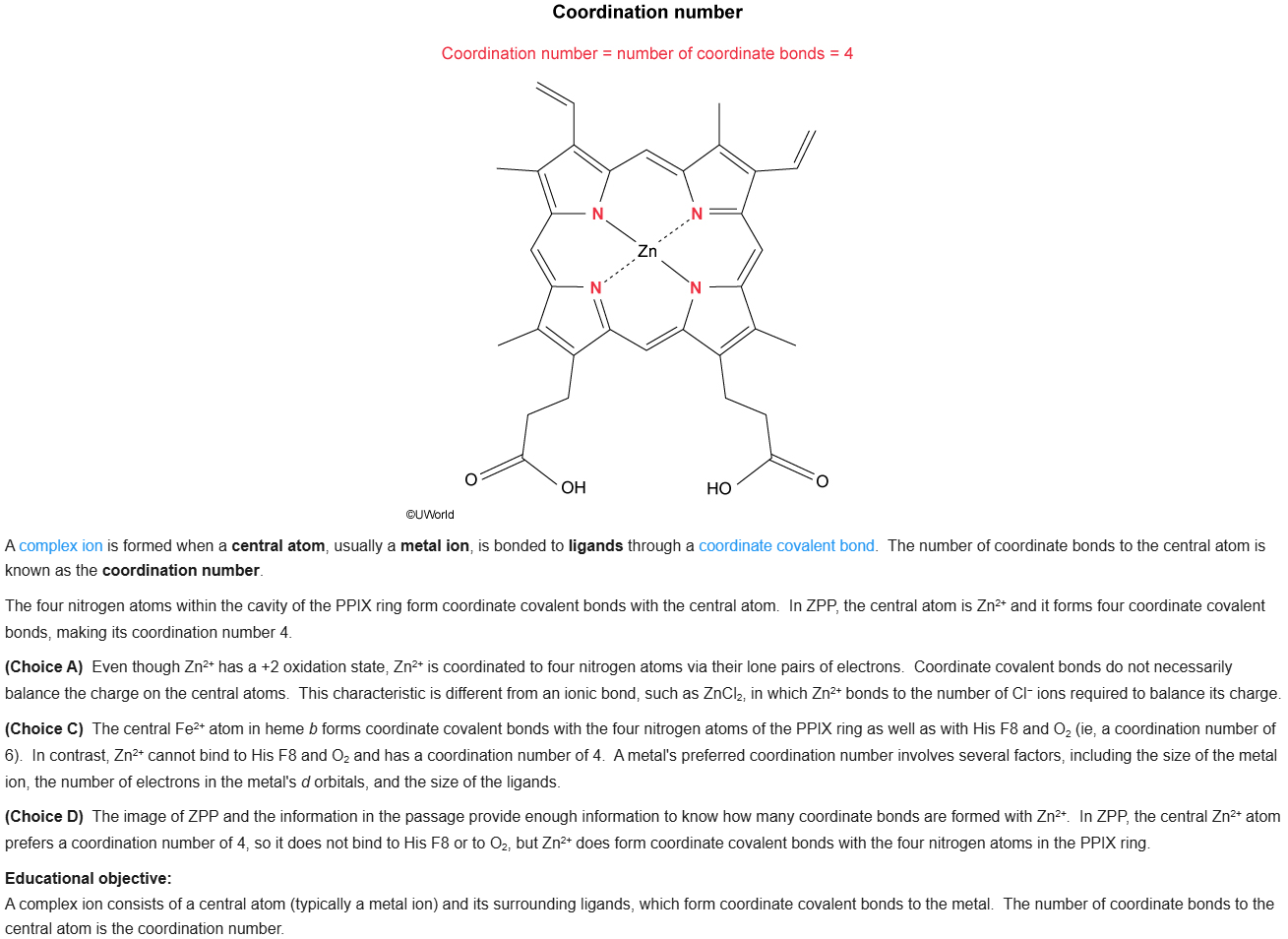
75
New cards
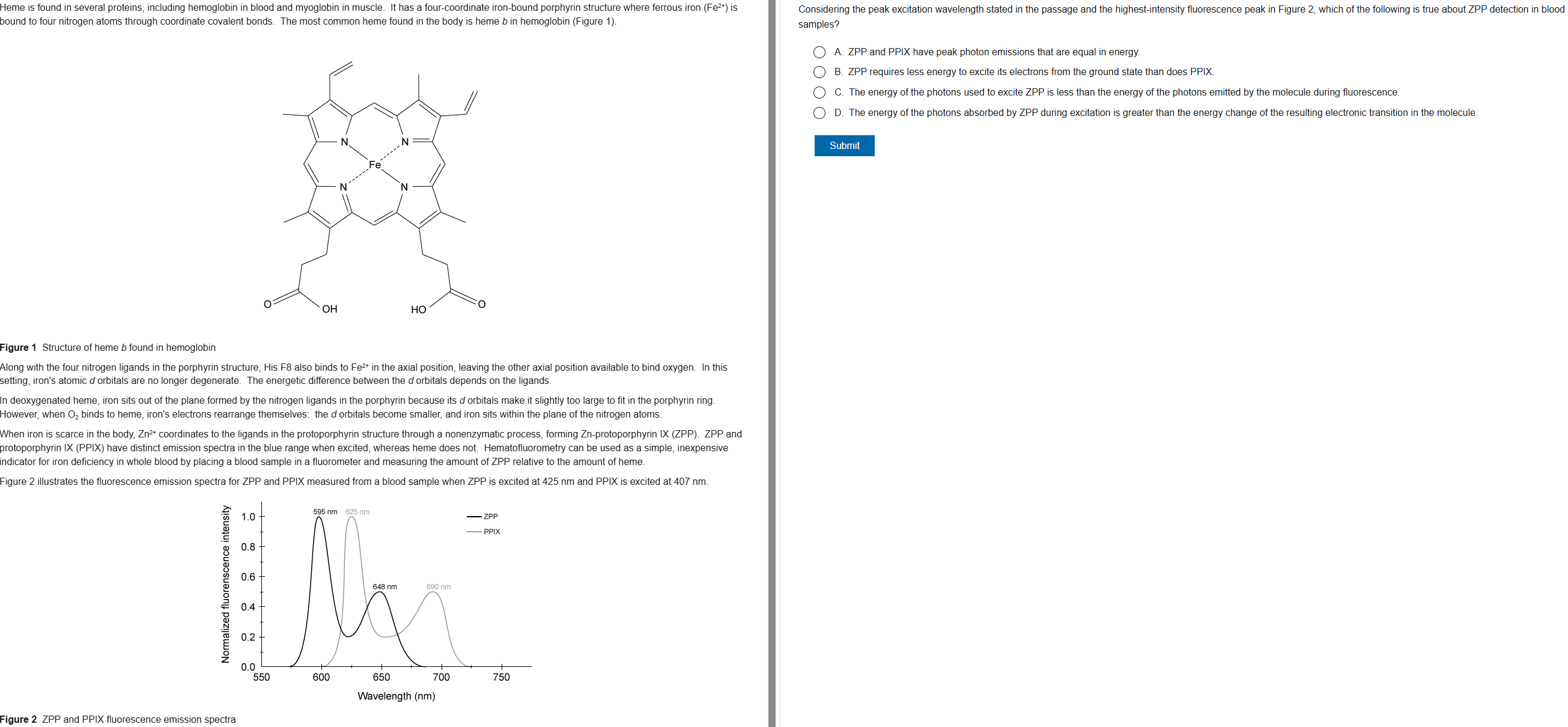
B
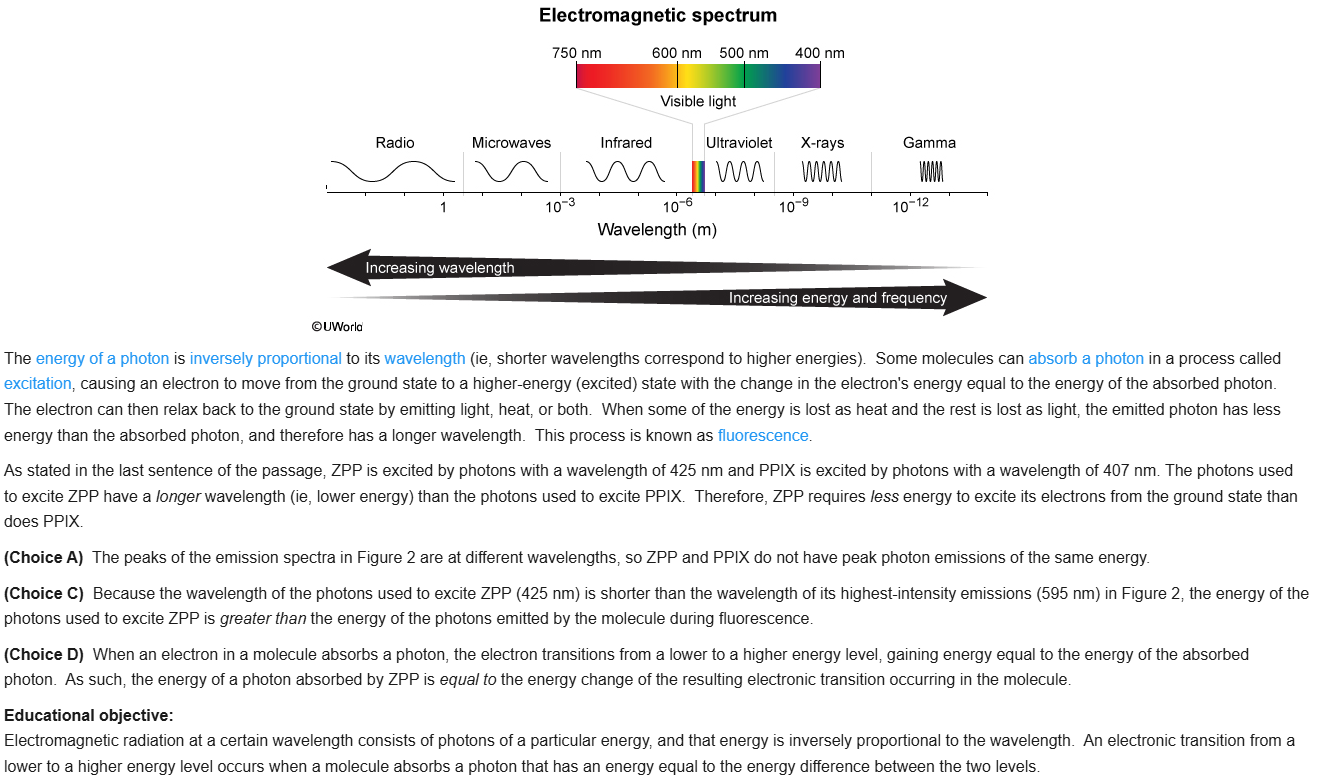
76
New cards

D
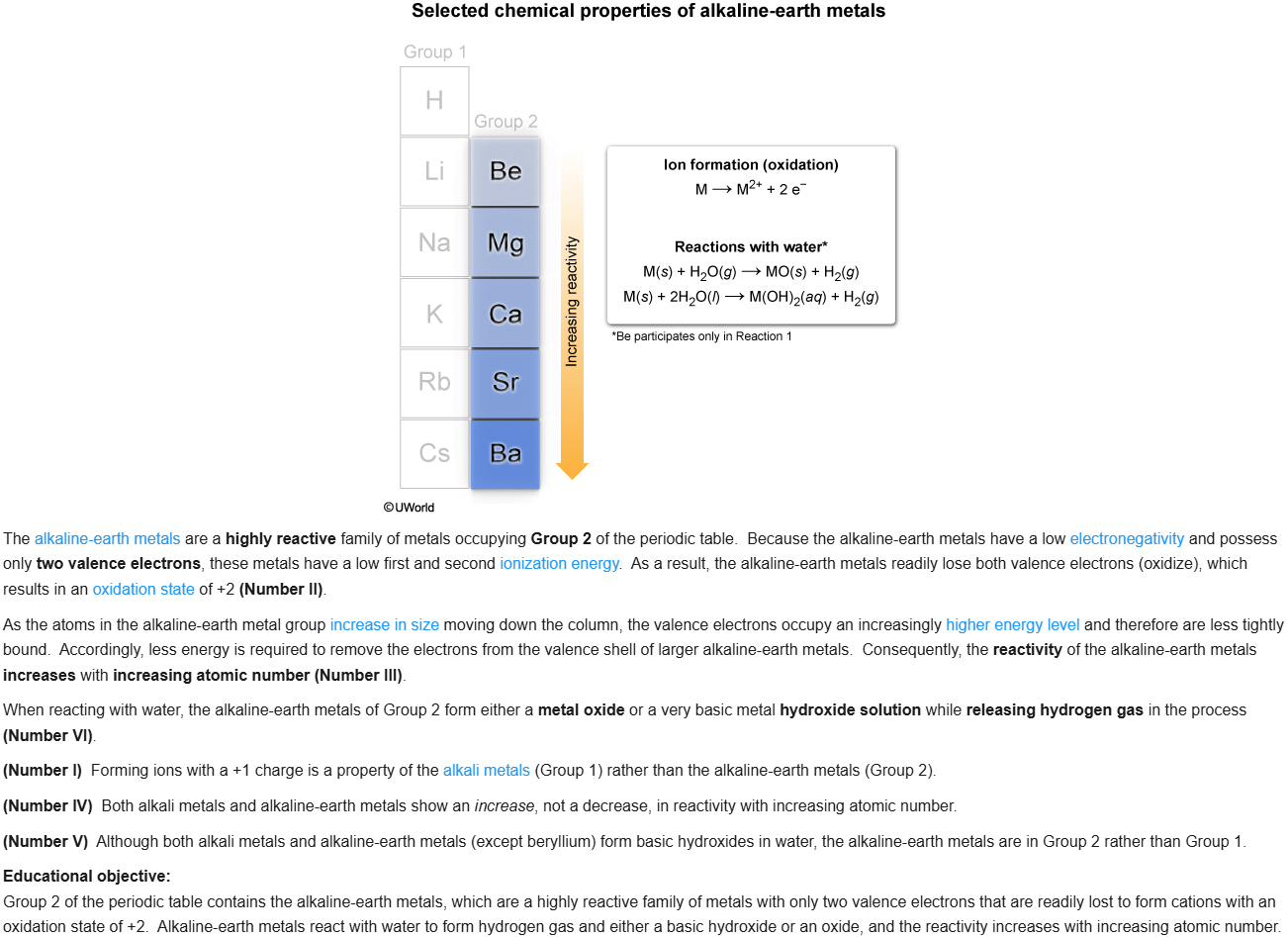
77
New cards
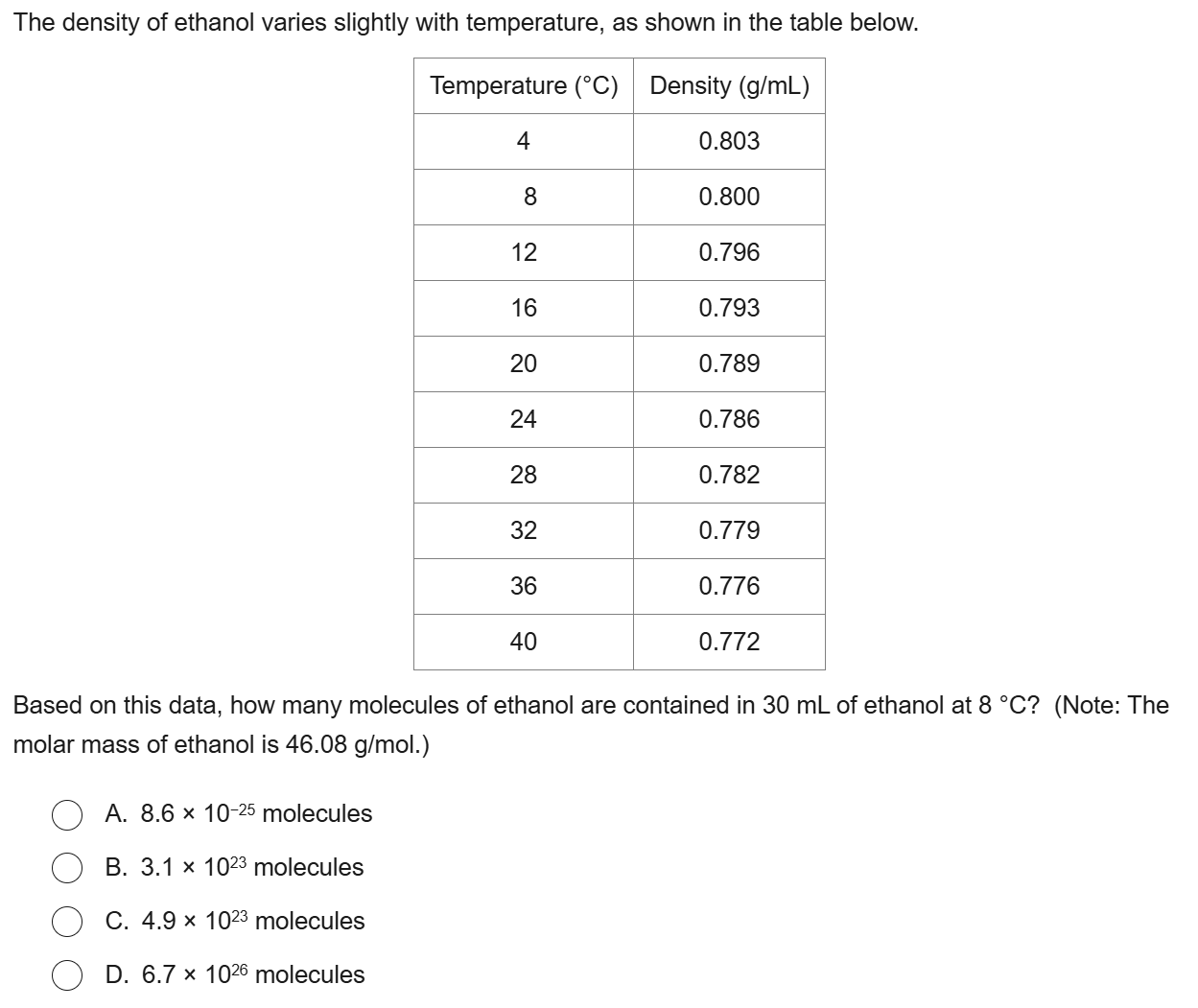
B

78
New cards

B

79
New cards
B
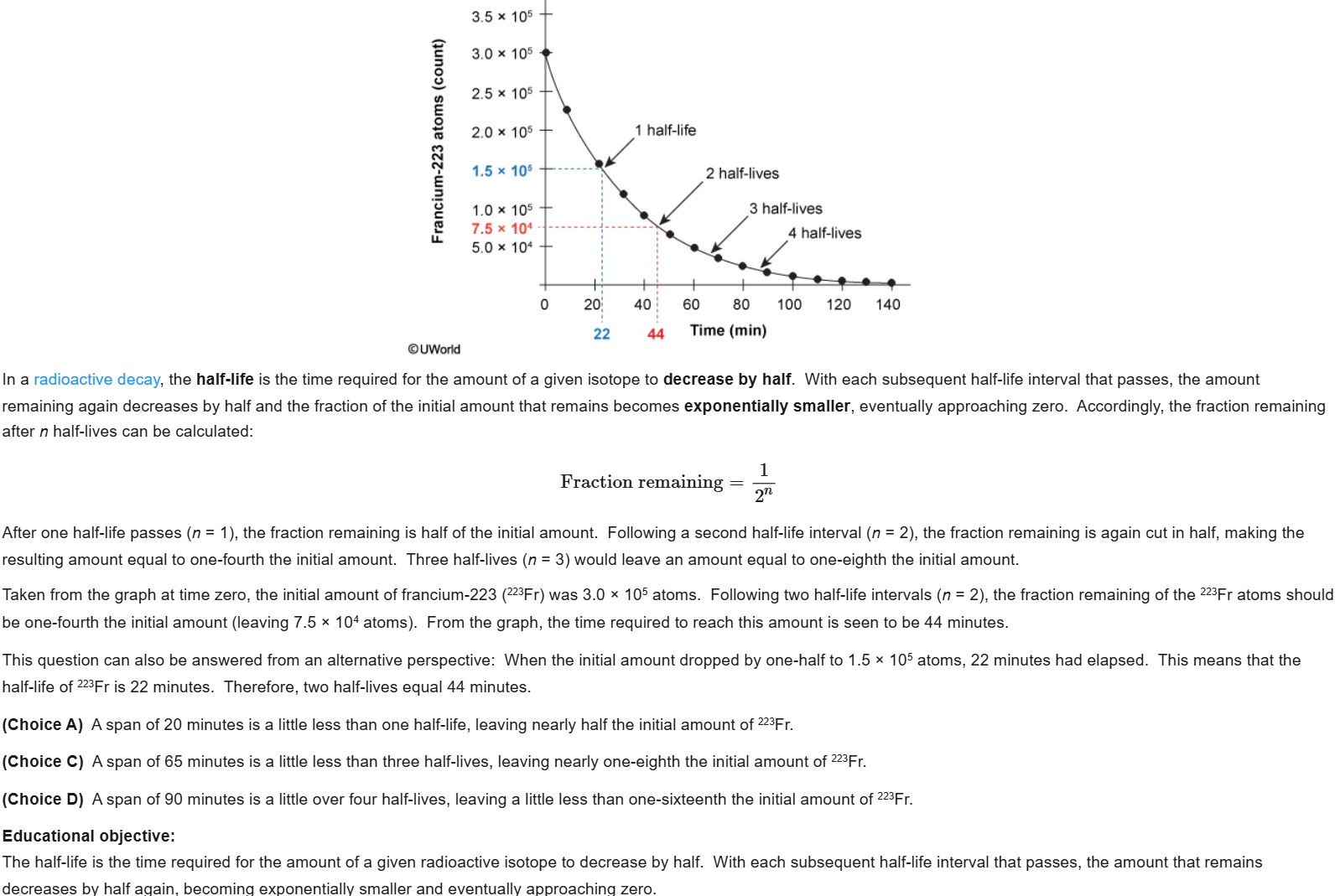
80
New cards
D

81
New cards
A
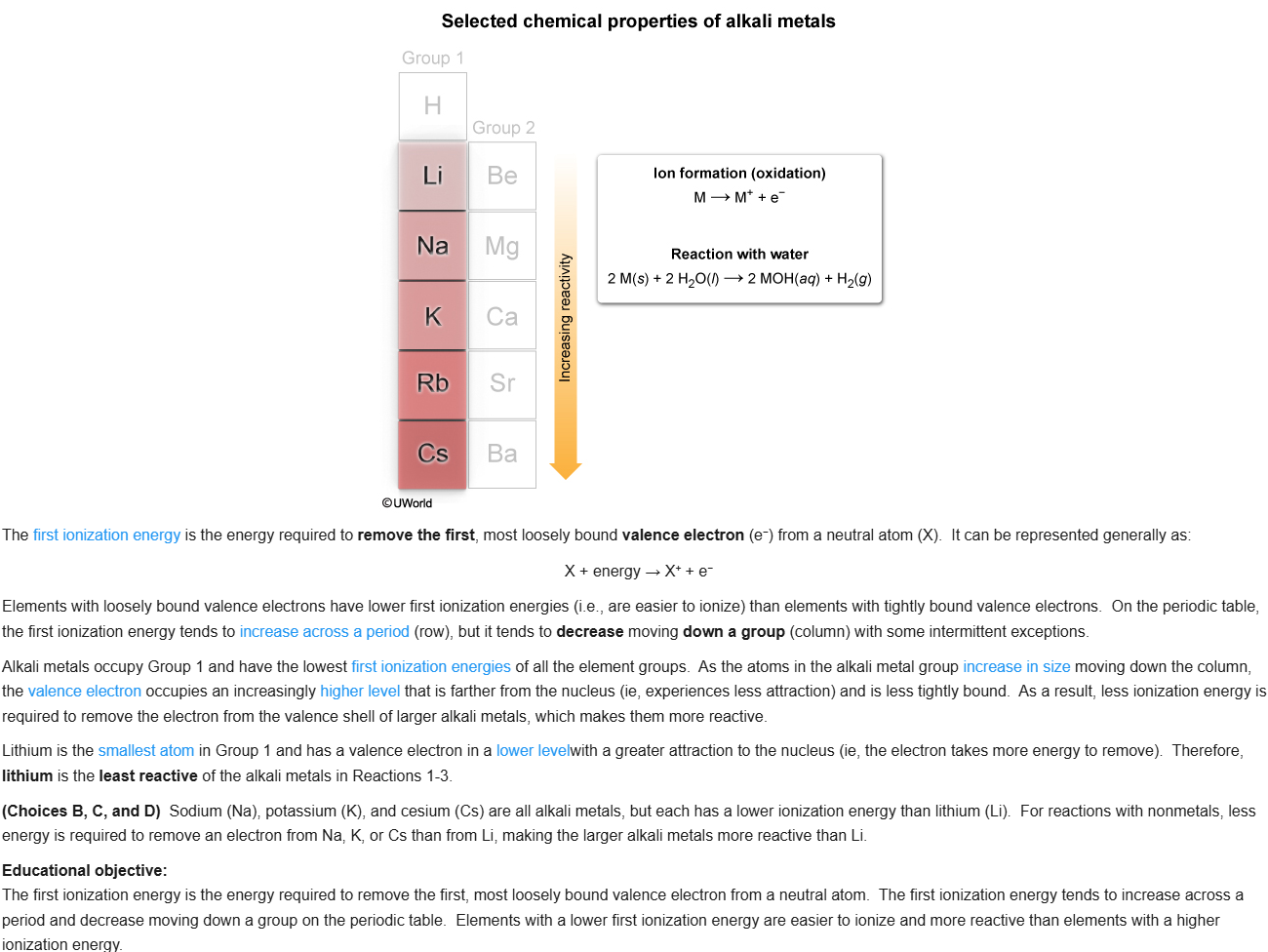
82
New cards

D

83
New cards
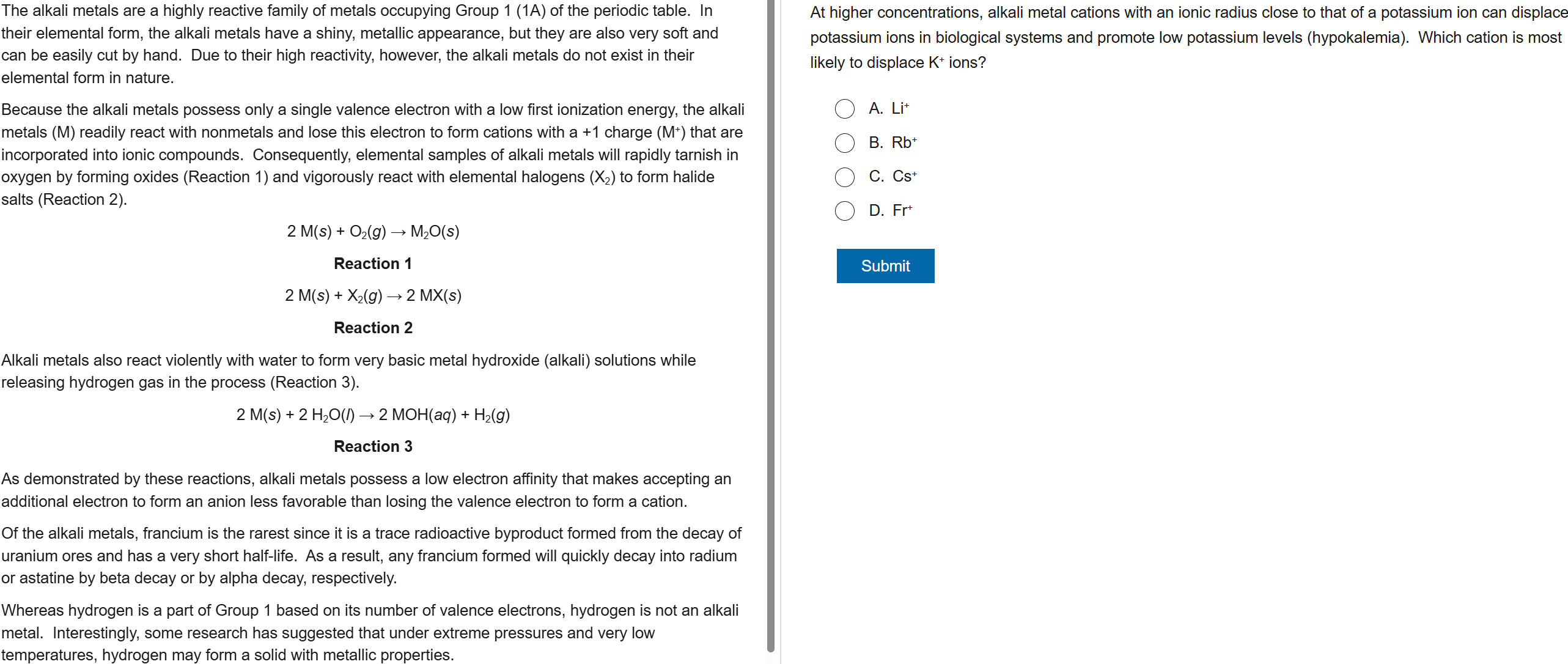
B
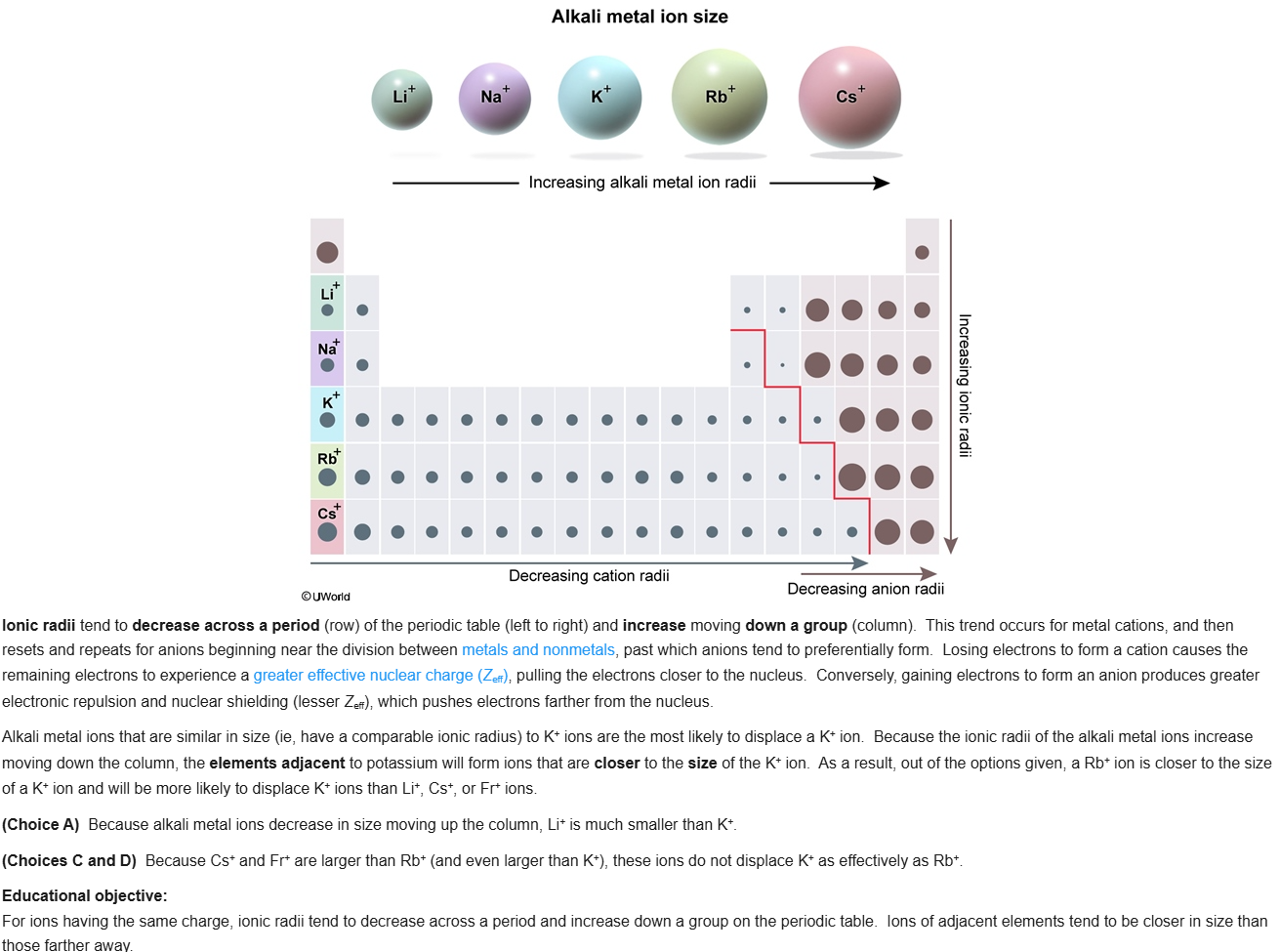
84
New cards
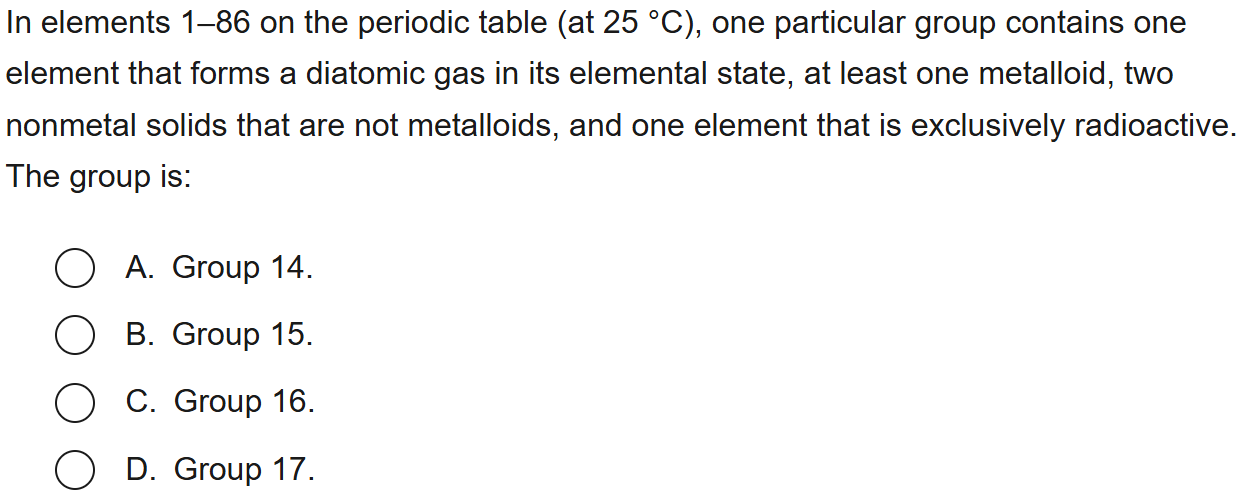
C

85
New cards
D
86
New cards
B
87
New cards
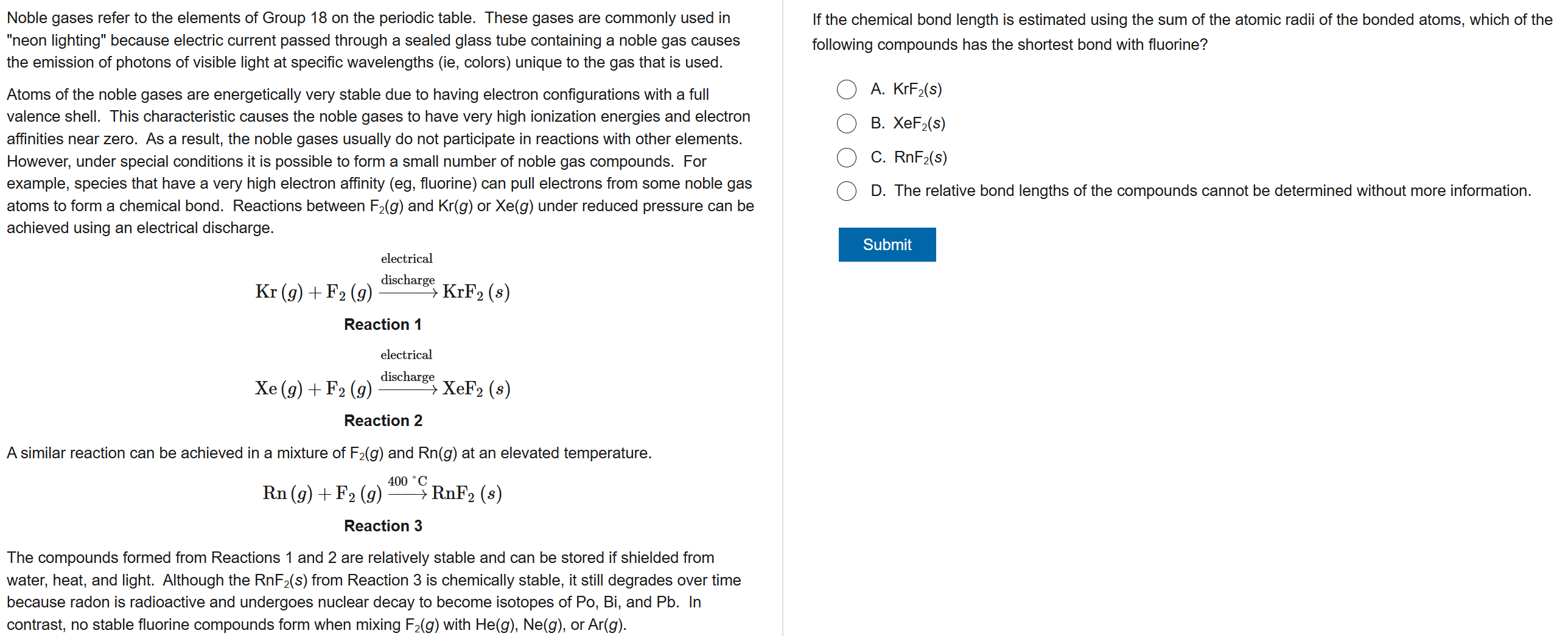
A

88
New cards
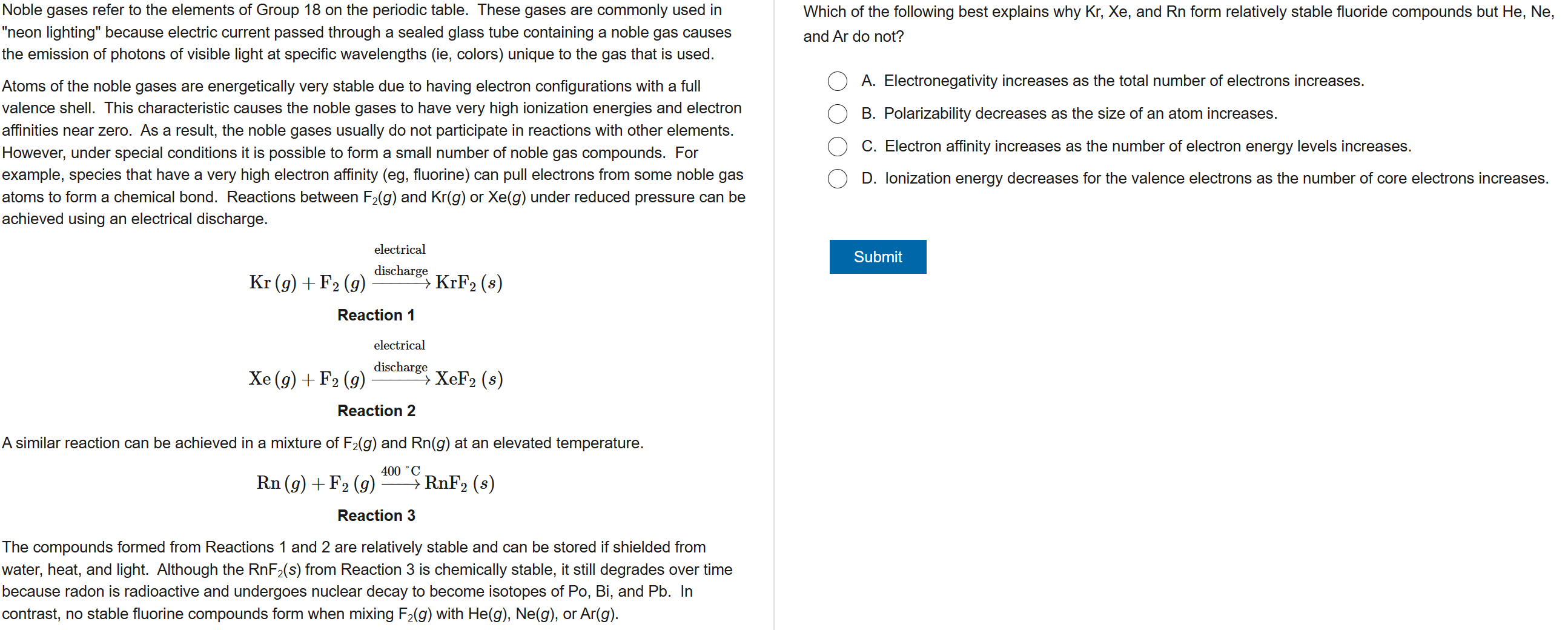
D
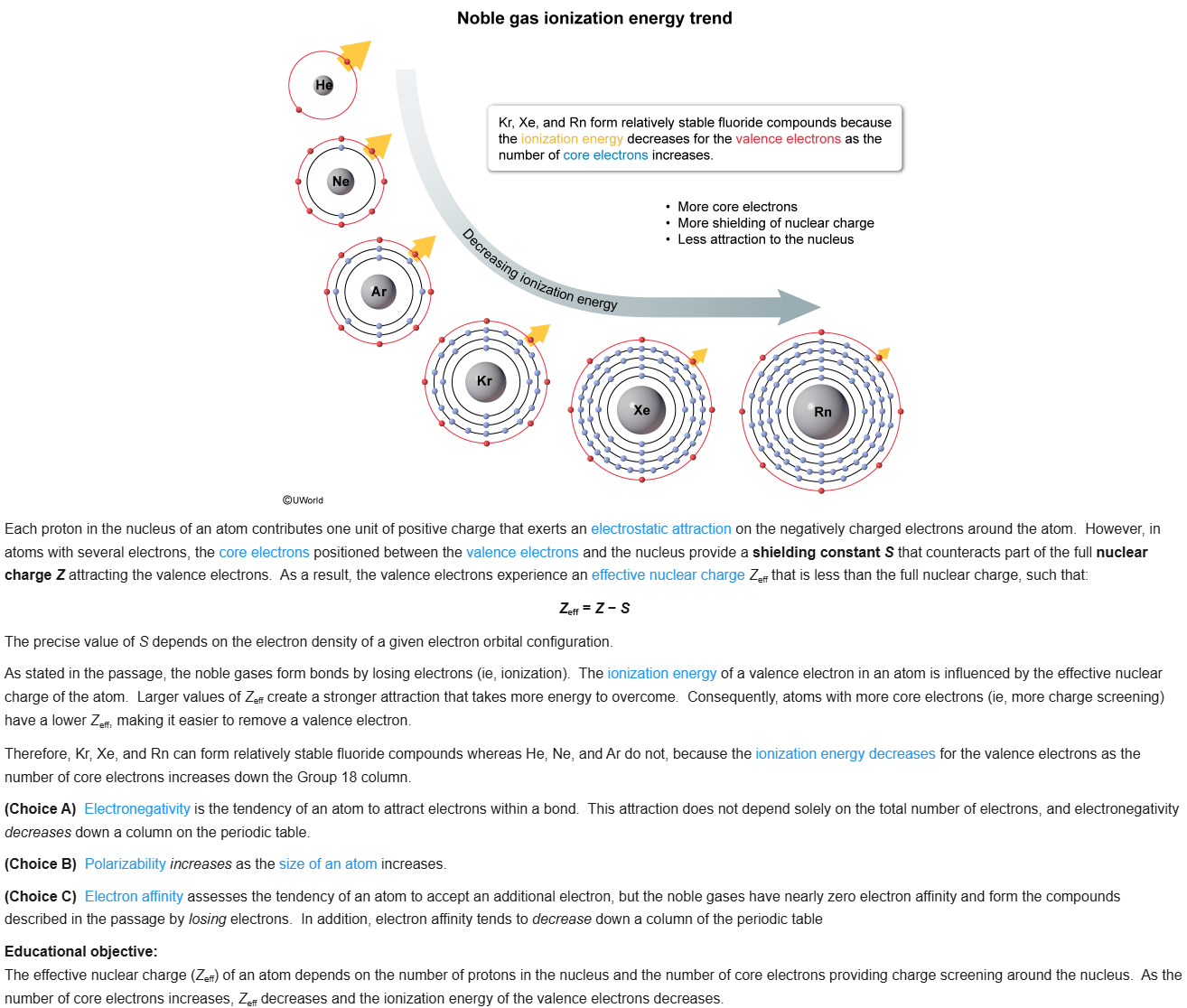
89
New cards
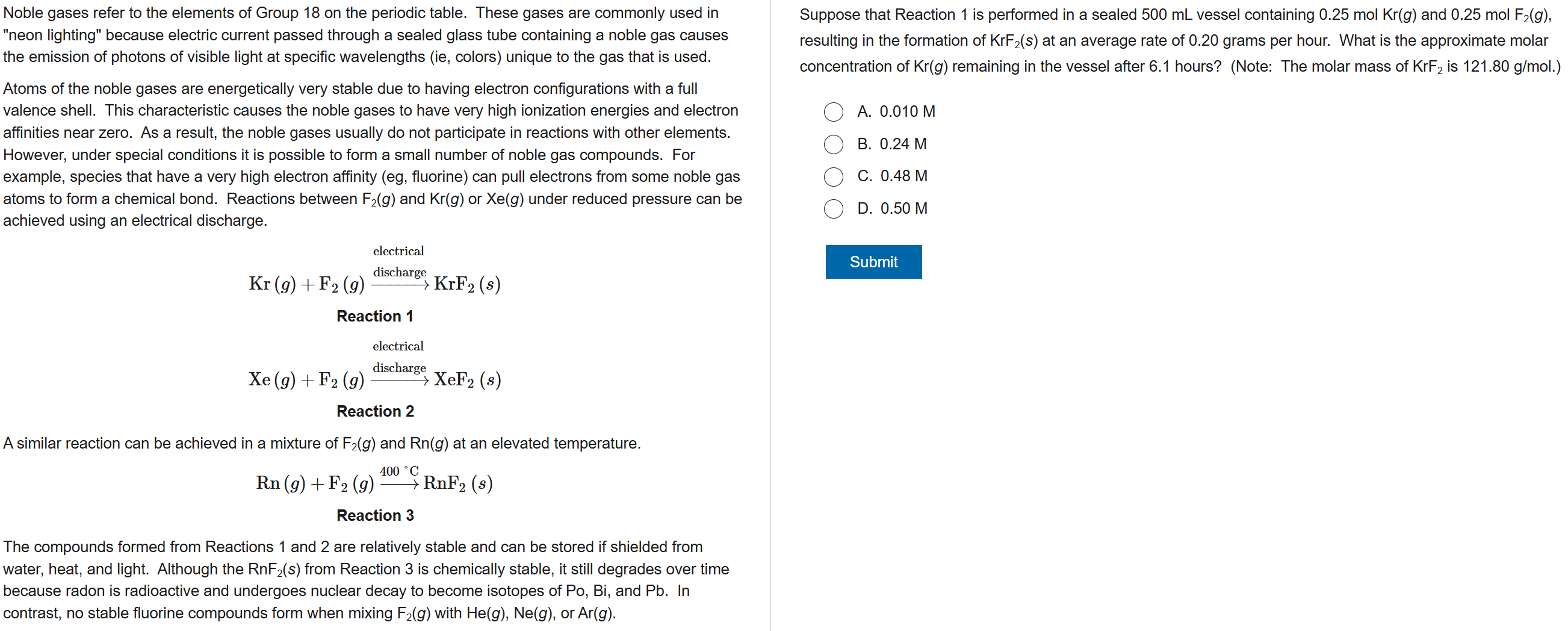
C

90
New cards
B
91
New cards
D
92
New cards

C
93
New cards

A
94
New cards
A
95
New cards
C
96
New cards
C

97
New cards
C
98
New cards
C
99
New cards
B

100
New cards

B