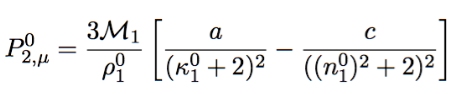Dipole moment
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
polarisation
charge distribution induced by ext. electric field; electric dipole moment per unit volume
Electron/distortion polarisation
displacement of electron charge relative to nucleus
orientation polarisation
arise from permanent dipole moment; boltzmann distribution
induced dipole
can pull electrons away from nucleus with a very strong field
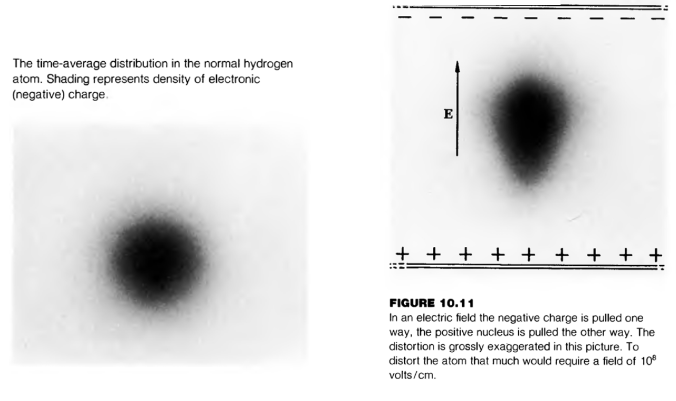
permanent dipole
center of negative charge doesn’t match center of positive charge even in absence of ext. field, don’t line up at ordinary temp./el. fields
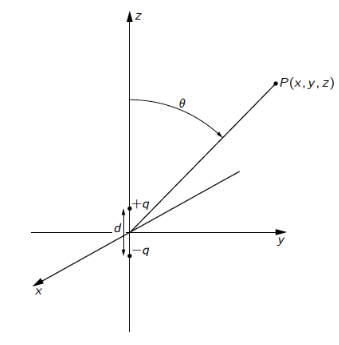
dipole approximation
described by two point charges (+ & -) separated by distance d
potential of dipole
q = charge
r = distance from point of measurement
proportional to qd; inverse to 1/r²
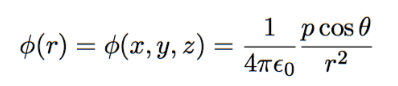
field of dipole
derivative of potential; proportional to qd; inverse to 1/r3
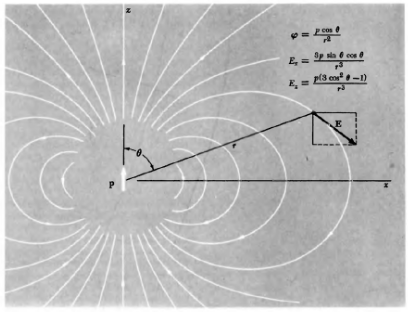
dipole moment
p=qd
atomic polarisability
how easy it is to induce dipole
p = αElocal in isotropic medium
α is a tensor/matrix in anisotropic mediums
isotropic medium
any direction appears the same; uniformity
anisotropic medium
direction dictates outcome
Clasusius-Mossotti equation
P = Np/V = NAρp/M
P = molar polarisation
N/V = # molecules/volume
NA = Avogadros
M = molar mass
ρ = density
Electric field in medium
local electric field as centre of spherical cavity in dielectric medium; addition of external and local field

total polarizability
distortion = α0
orientation = μ²/3kT
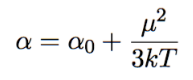
molar polarizability
distortion = α0
orientation = μ²/3kT
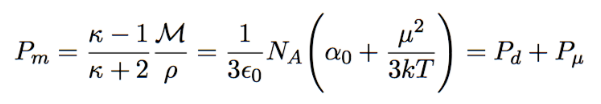
molar refraction
index of refraction relates to dielectric constant by κ = n²
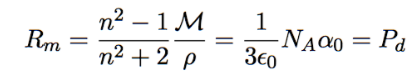
measurement in solution
solute and solvent contribute to properties; dipoles interact with each other & solvent; solvent has disortion polarisability; use dilute solutions → extrapolate to infinite dilution

hedestrand method
assume linear dependence on mole fraction
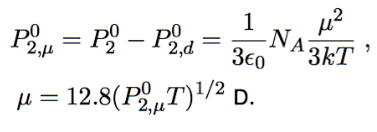
Smith-Guggenheim method
n²= (n10)² + cX2