week 1: ambient air pollution & health effects
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
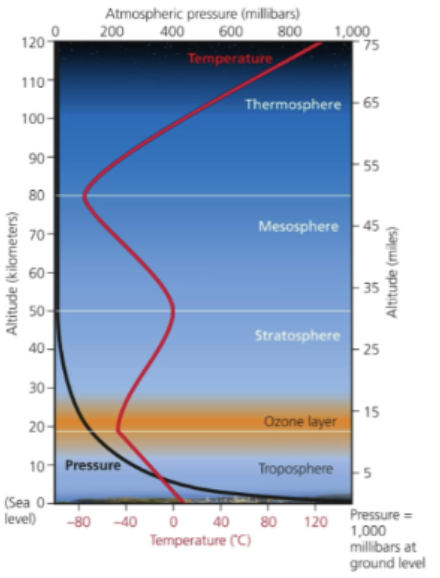
ozone (O3)
layer in the stratosphere, thinnest in the tropics (around the equator) and denser towards the poles
at the ground level, ozone is a health hazard
major component of photochemical smog
common in suburban regions downwind from cities
stratospheric level ozone
forms a protective layer that serves a vital function: it absorbs the wavelength of solar radiation aka UV-B (ultraviolet-b)
sunburn, skin cancer, eye damage, cataracts aka eyes (estimated 10% reduction ozone layer → 25% increase non-melanoma skin cancer
suppress immune system
DNA mutation
ozone depletion contributors
chloro-fluoro-carbons (CFC)
used as refrigerants (notably in air conditioners), as agents in some manufacturing processes, and formally as propellants in spray cans; takes 10-20 years to get to the stratosphere and 65-385 years to break down
halogen compounds
fire fighters used bromine-containing halogens to put out fires
in the stratosphere, intense UV radiation breaks the CFC molecules apart, which lead to…
releasing the chlorine acts as a catalyst (takes part in several chemical catalyst) yet at the end emerges unchanged and is able to react again
1 chlorine atom average destroys
1,000 ozone molecules before converted into form harmless to ozone
1987 montral protocol on substances
first global agreement to restrict CFCs and deplete ozone layer
acid rain
reactions in the atmosphere and can fall many miles from where the pollution originated
Oxides of sulphur and nitrogran produce acid aerosol - H2SO4, HNO3
Health Effects of Acid Rain
Impaired forest growth → reduced ecosystem productivity
Increase in acidity for lakes and rivers
Death of fish and wildlife
Weathering of monuments and buildings
Health impacts for those prone to respiratory ailments
Trans-boundary issue
pollutants reaching higher altitudes may be transported thousands of km, so areas with little to no local industry can still experience high levels of toxic contamination
smog
industrial smog (burning sulfur-rich oil or coal)
photochemical smog (sunlight reaction to pollutants aka brown air smog; tropospheric ozone)
atmospheric pressure low to high
london killer smog of 1952
Average smoke and burning culter rich oil or coals and the relationship to death for 12 London sites
Peak in the number of deaths coincided with the peak in both smoke and pollution levels
1970
earth day
Clean Air Legislation
Air Pollution Control Act (1955): first federal air pollution law, identifying air pollution as a national problem
Clean Air Act (1963): first national air pollution control that set emissions standards for stationary sources
Clean Air Act (1970): added identification of critical pollutants and established ambient air quality standards; A full list of 188 hazardous air pollutants targeted
Revision (1990): provision for acid rain, urban smog, toxic air pollutants, ozone protection, marketing pollution rights, fugitive emissiosof voltile organixs, ambient ozone, soot, and dust, and NOx emmisions
clean ari act 1970 revised
CRITERIA POLLUTANTS
Primary standards:
Protect public health
Secondary standards:
Protect public welfare
AIR TOXICS
No primary or secondary standards that limit pollutant concentrations in the air
Maximum achievable control technology: reduce emissions from specific industrial "source categories" (like chemical plants, oil refineries, or steel mills)
6 critical common air pollutants
Particulate matter
Carbon monoxide
Nitrogen oxided
Ground level ozone (smog)
Lead
Sulfur dioxide
nonattainment
failure to meet the standards
by law, EPA must review the 6 critical pollutants every…
5 years
gaseous, aerosol, or particulate pollutants other than the 6 critical pollutants except for lead…
are present in air in low concentrations, but are toxic and persistent
health effects of ozone
chest pain
coughing
throat irritation
congestion
worsen bronchitis
asthma
emphysema
reduce lung function and inflame the linings of the lungs
permanent scarring
lung cancer
Nitrogen Dioxide (NO2)
gaseous products of combustion from both stationary sources and from motor vehicles
in contrast to ozone, nitric oxide is found at higher levels indoors than outdoors
Health Effects of Nitric Oxide
increased respiratory tract symptoms, reduced lung function from direct inflammation increased respiratory tract infection from effects of defence mechanisms.
deteriostion of health status of persons with chronic respiratory diseases
Sulfur dioxide and acid aerosols (SO2)
emitted by fossil fuel combustion (coal and oil fired power plants and industrical processes
Before the 1970 Clean Air Act, sulfur dioxide was primarily released from smoke stacks at relatively low heights within the ground-based mixing layer, so it reacted quickly to deposited on the ground on vegetation and surfaces
Limits placed on sulfur dioxide concentrations, power plants and other emission sources had tall stacks installed to reduce local ambient air concentrations
Particulate Matter
classified by their size (mass median aerodynamic diameter
PM10: <10 μm
PM2.5: <2.5 μm
Ultrafine PM (UFP): <0.1 μm (mass infinitesimal for UFP, but number enormous)
Health Effects of Particulate Matter
heightened ambient levels of PM2.5 lead to respiratory illnesses and cardiovascular endpoints, and ultrafine can penetrate cell walls and the blood-brain barrier which leades to organs absorbing it
Carbon Monoxide (CO)
reacts with blood hemoglobin
Health Effects of Carbon Monoxide
tissue hypoxia, organs with high metabolic rates like CNS and the cardiovascular system
high levels lead to death
low levels lead to cardiovascular & neurobehavioral (headaches/nausea/fatigue)
Vulnerable population like fetus, infants, people with anemia or chronic heart or lung disease, high altitude residents, smokers
Current and Future Conditions
In the U.S. air quality has improved in the last deceased, exepct for areas that still sever traffic and wildfires
Air quality is poor in developing countries
Air pollution in 2023
2nd risk factor for mortality worldwide
How does air pollution worsen COVID-19 outcomes
Decreases immunes system function to fight viruses
Causes inflammation and oxidative stress in lunges
Can lead to severe pneumonia
Cardiovascular and metaboli csystem can worsen
What are the two major air pollution problems at global level?
1) Ozone Depletion: CFCs and Halogen compounds destroying the ozone layer (stratospheric ozone). UV radiation breaks apart CFC molecules and release chlorine atoms which in turn reacts with ozone and destroys it
2) Climate change: carbon dioxide and methane emission levels are harder to treat than just having a substitute for ozone layer
What are the two major air pollution problems at regional level?
1) Acid Rain: pollutants (oxides of sulfur and nitrogen) produce acid aerosols that can travel long distances
2) Photochemical smog: "smoke" + "fog" industrial smog and photochemical smog (triggered by UV rxn with pollutants)
What are the major air pollution problems at local level?
1) Nitrogen Dioxide (NO2): high levels indoors than outdoors, gas products of combustion from stationary sources and motor vehicles
2) Sulfur dioxide and acid aerosols: SO2 emitted by fossil fuel combustion (coal, oil)
3) Particulate matter (PM): fine particles getting inhaled and penetrated
4) Volatile Organic Compounds (VOCs): volatile and readily inhalable (ex: butane, propane, octane) emitted naturally by decomposition and industrial activities, precursors of smog
5) Carbon Monoxide (CO): reacts with blood hemoglobin
ozone
good up (statosphere), bad nearby (troposhpere)
voc
volatile organic compounds from vegetation and industrial sources + nitrogen oxide from automobiles + power plants + sunlight = ground level ozone
why does particulate matter PM size matter
High ambient levels of PM 2.5 lead to mortality, respiratory illness, and cardiovasuclar endpoints
UFP can penetrate cell walls and the blood-brain barrier and can be easily absorbed into vital organs
The bigger the number (10) = larger particle
The smaller the number (2.5)
The size of the particle determines how far and what it penetrates into the body
in which atmospheric region does the ozone layer occur and what would be the human health consequences of ozone layer destruction?
The ozone layer occurs in the stratosphere and can be destroyed by man-made chemicals
Human health consequences: sunburn, skin cancer, eye damage, cataracts, suppresses immune system, DNA mutation
on an overcast or cloudy day, would you expect any O3 buildup due to smog? Explain why.
no, would not expect any O3 buildup due to smog. Photochemical smog relies on UV radiation in order to be created.
what international treaty went into effect in 1987, aiming at mustering worldwide effects to protect the ozone layer? What is the major action called by this treaty?
1987 Montreal Protocol on Substances that deprete ozone
First global agreement to restrict chloro-fluoro carbon (CFC)s and try to find alternative to CFC products
7. Which geographical area in the U.S. has the most significant acid rain problem and what are the environmental and human health effects of acid rain?
1) The Northeastern United States has the most significant acid rain problem.
This is because of the large number of cities, the dense population, and the concentration of power and industrial plants in the Northeast.
2)
- Impaired forest growth reduced ecosystem productivity
- Increase in acidity for lakes and rivers
- Death of fish and wildlife
- Weathering of monuments and buildings
- Health impacts for those prone to respiratory ailments.
What are the differences between industrial smog and photochemical smog?
1) Industrial smog:
-Burning sulfurrich oil or coal creates SO2, SO 3, sulfuric acid, ammonium sulfate.
-Carbon leads to CO2 and CO.
-Days with stagnant air, usually winter.
2) Photochemical smog:
-Smog from reaction of sunlight with pollutants
-The kind that blankets so many American cities today
-"Brown air smog"
-Contains tropospheric ozone, NO2, VOCs, 100 more...
-Hot sunny days in urban areas create perfect conditions.
Why is O3 presence in the troposphere a paradox to O3 in the stratosphere?
Good up, bad nearby
In the stratosphere (good): Ozone forms the "Ozone Layer". Its chemical role here is to absorb the sun's ultraviolet (UV) light. Without this "good" ozone, life on the surface would be sterilized by radiation
In the Troposphere (bad): Near the ground, ozone is a toxic gas. because it is highly reactive, it oxidizes (chemically burns) lung tissue when inhaled and damages the cells of plants, reducing crop yields.11 Here, it is the primary ingredient of smog.
what does VOC stand for? What is the role of VOC in smog formation?
Volatile organic compounds from vegetation and industrial sources + nitrogen oxide from automobiles + power plants + sunlight = ground level ozone
VOCs are volatile and readily inhalable ( butane, propane, and octane)
VOCs are precursors of photochemical smog and react with nitrogen oxides emitted from vehicles, power plants and industrial activities to form ozone, which in turn helps the formation of fine particulates.
cite two ways in which hazardous air pollutants (HAPs) differ from criteria pollutants.
The criteria pollutants have primary and secondary standards.
The HAPs have no primary or secondary standards that limit pollutant concentrations in the air, but have maximum achievable control technology standards (MACT)
Particulate matter (PM) air pollution is sometimes categorized as PM10, PM2.5, or ultrafine particles. Why is particle size an important factor determining the adverse human health effects?
The size of the particles is related to their potential to cause health problems. Small particles, less than 10 micrometers, are the biggest problem because they can penetrate deep into the lungs, and some can even enter the bloodstream. Additionally, UFP can also penetrate cell walls and the blood brain barrier and can be absorbed into vital organs.