Gas Laws: Key Definitions and Equations for Chemistry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Charles' Law Equation
V1/T1=V2/T2
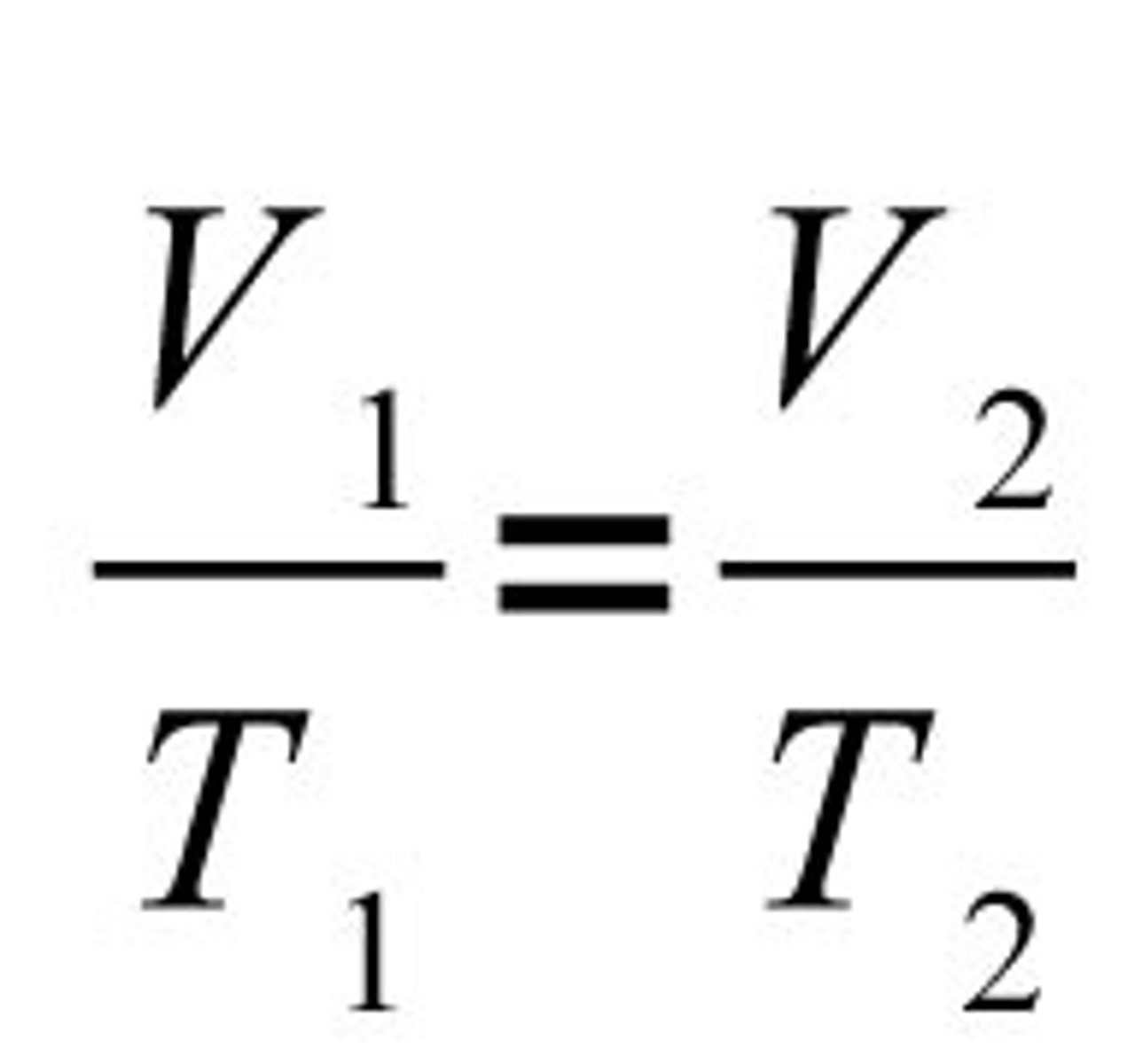
Gay-Lussac's Law Equation
P1/T1 = P2/T2
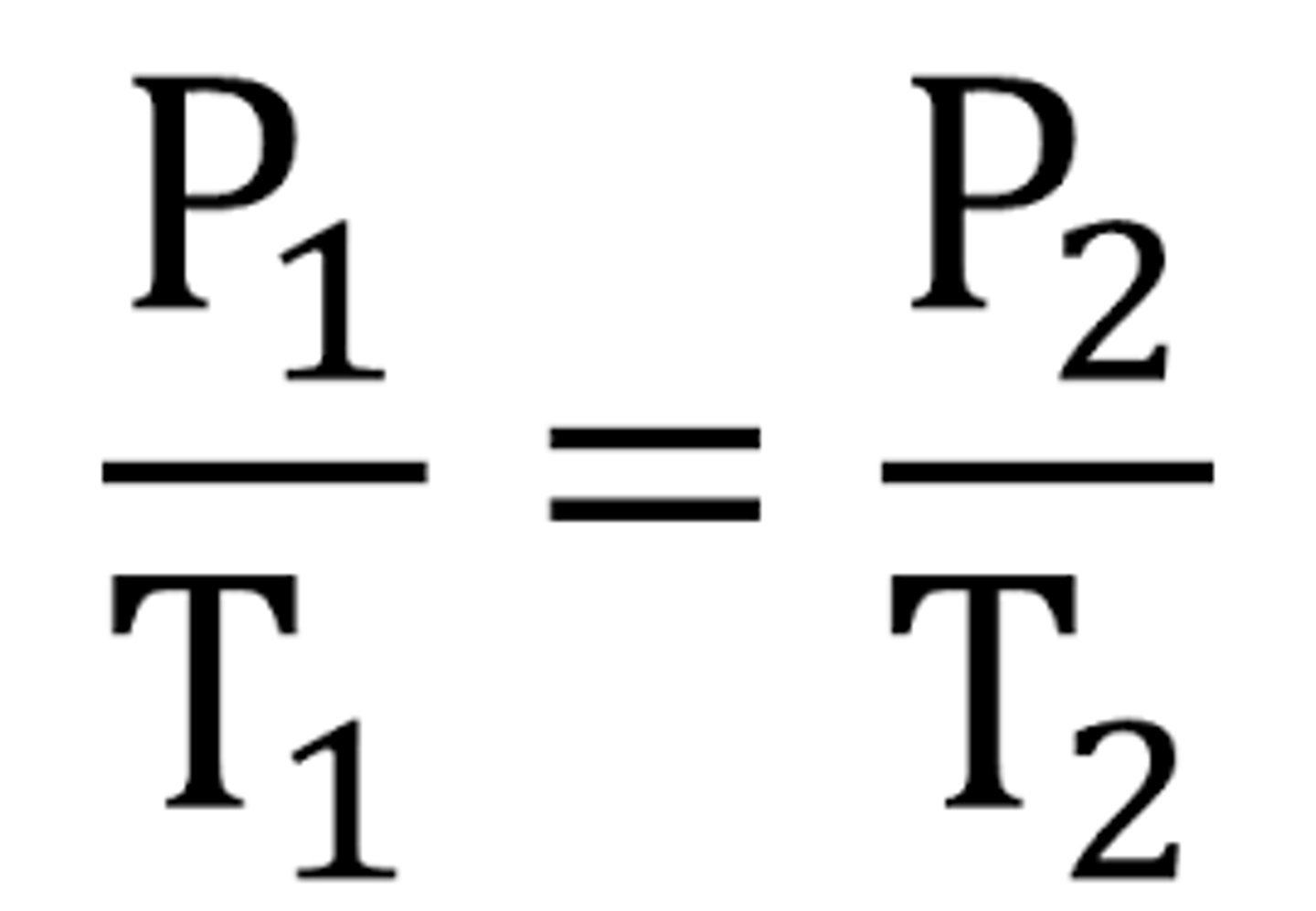
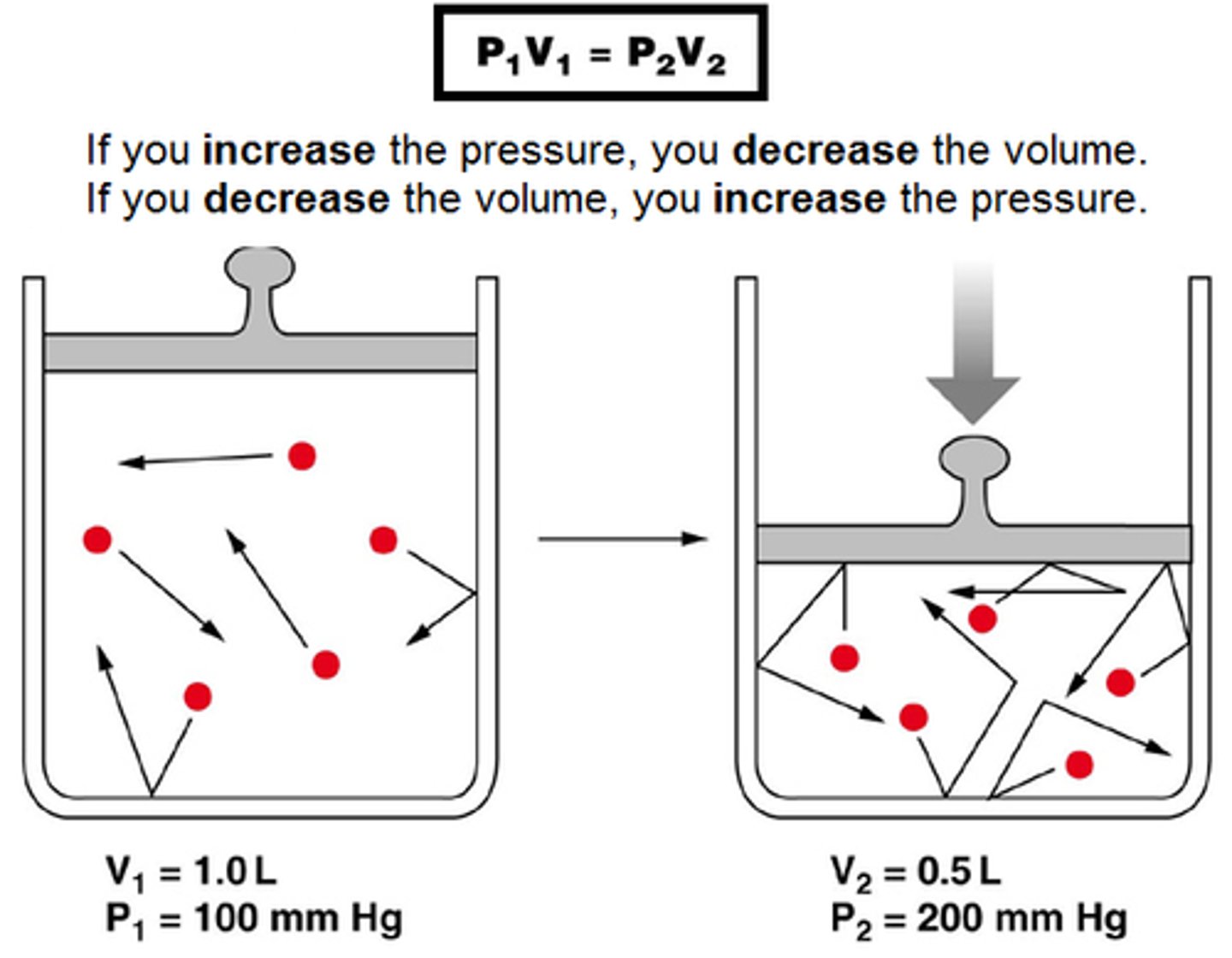
Boyle's Law Equation
P1 V1 = P2 V2
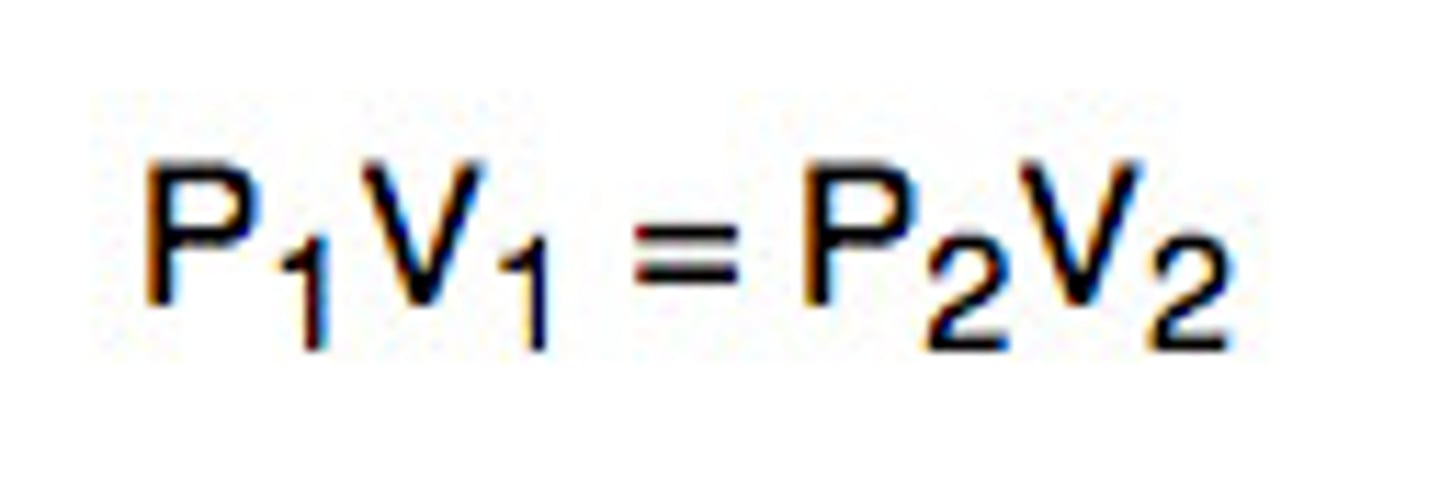
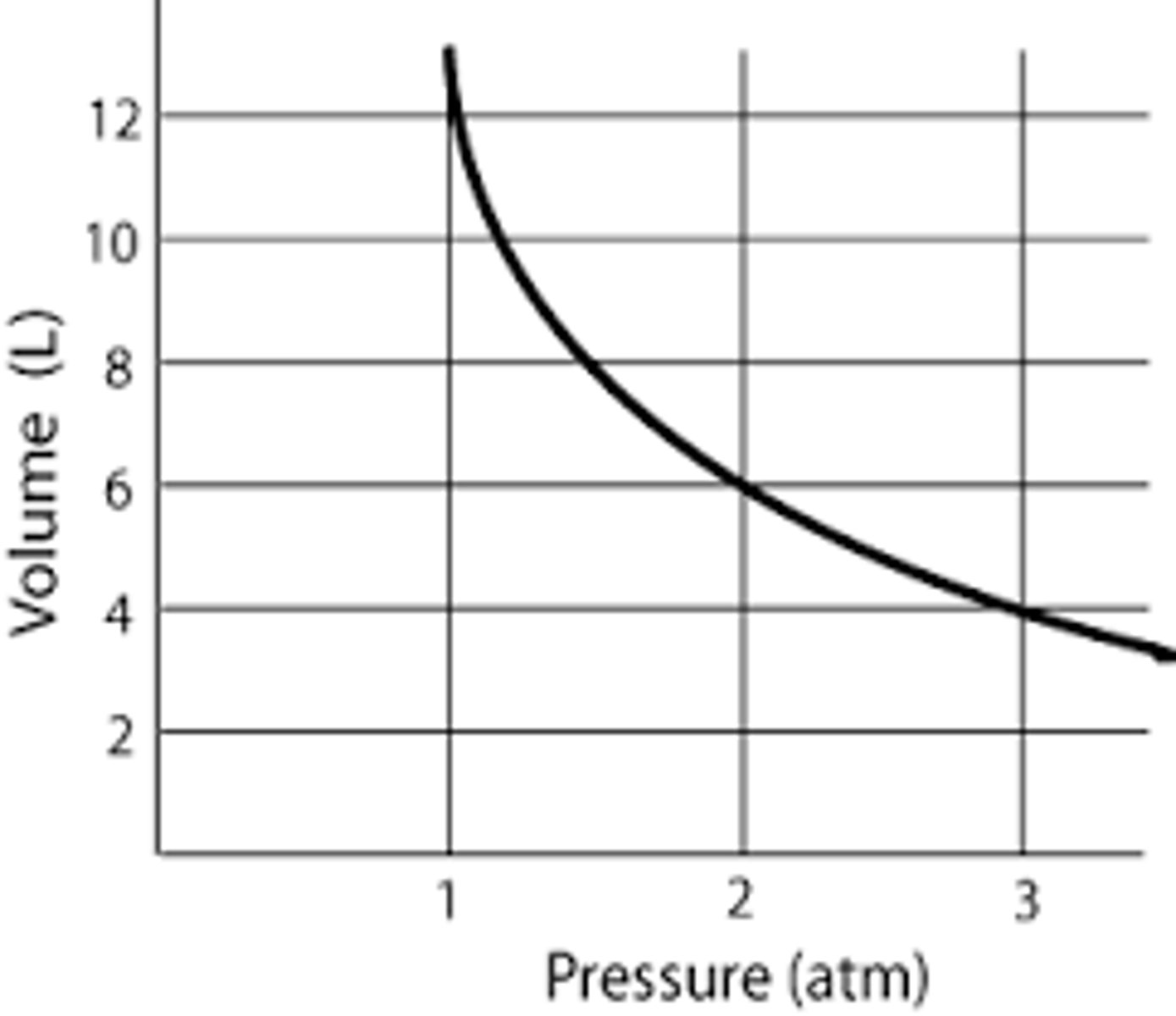
Boyle's Law Graph
Inverse relationship pressure and volume
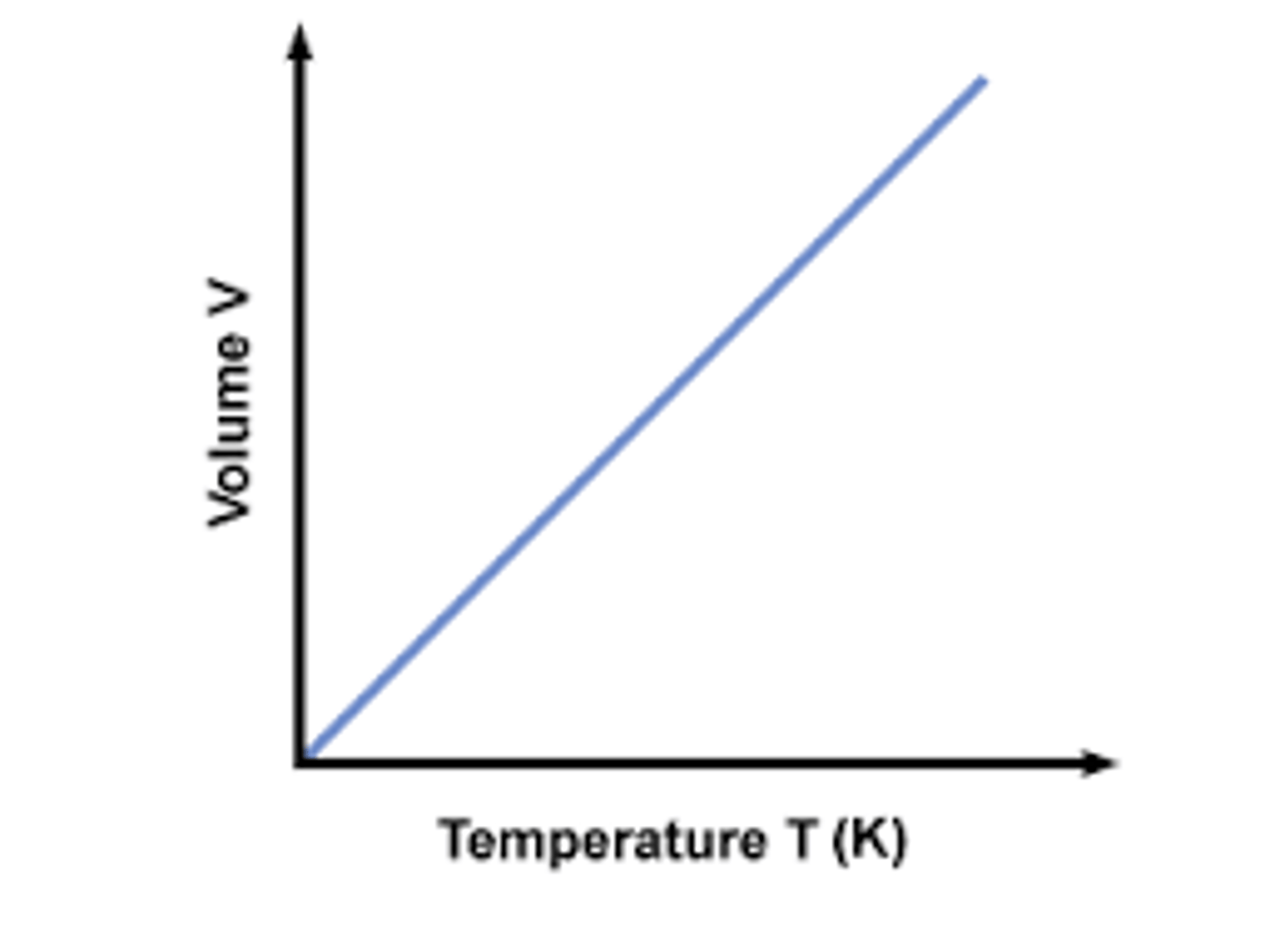
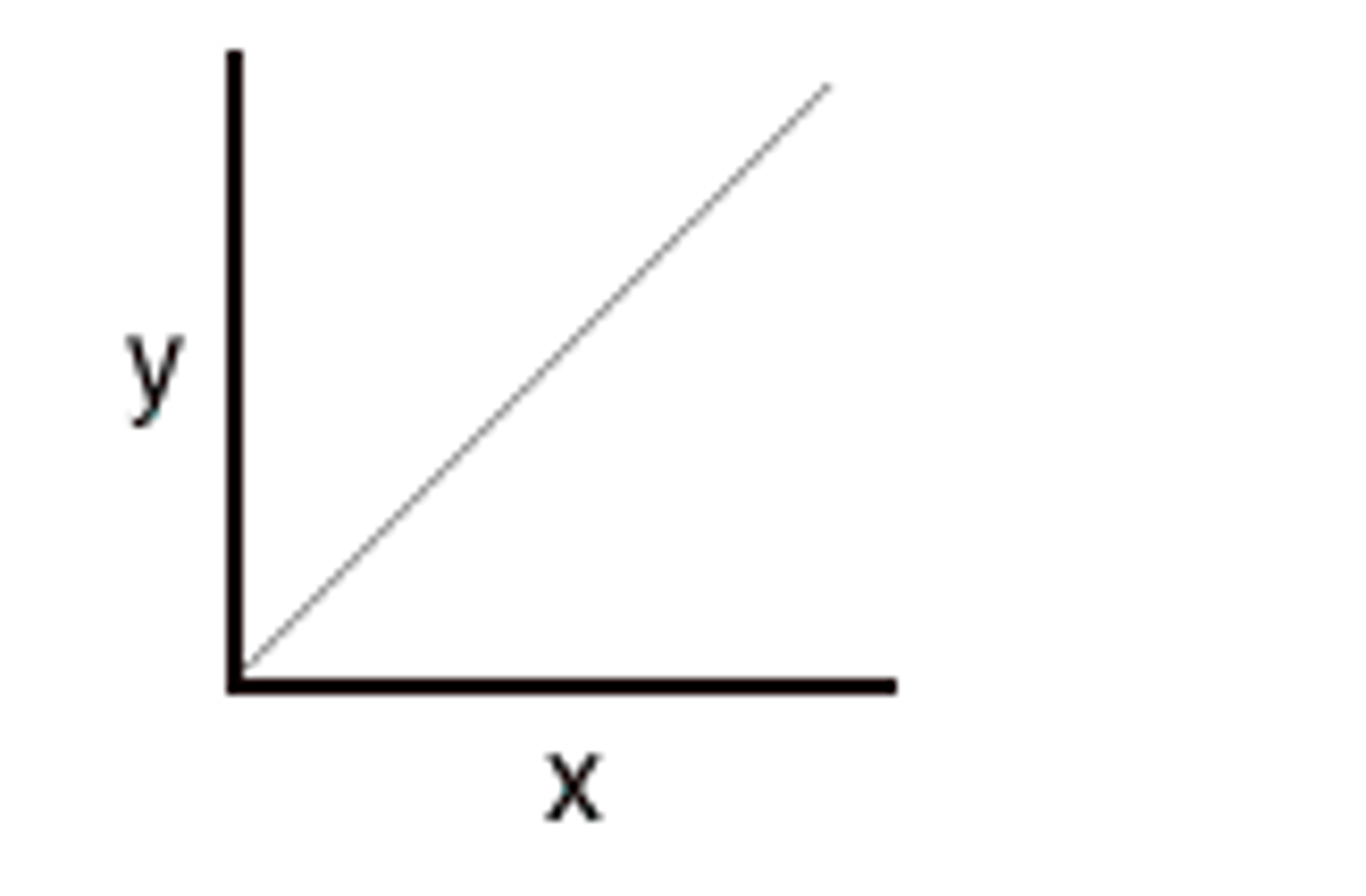
Charles' Law Graph
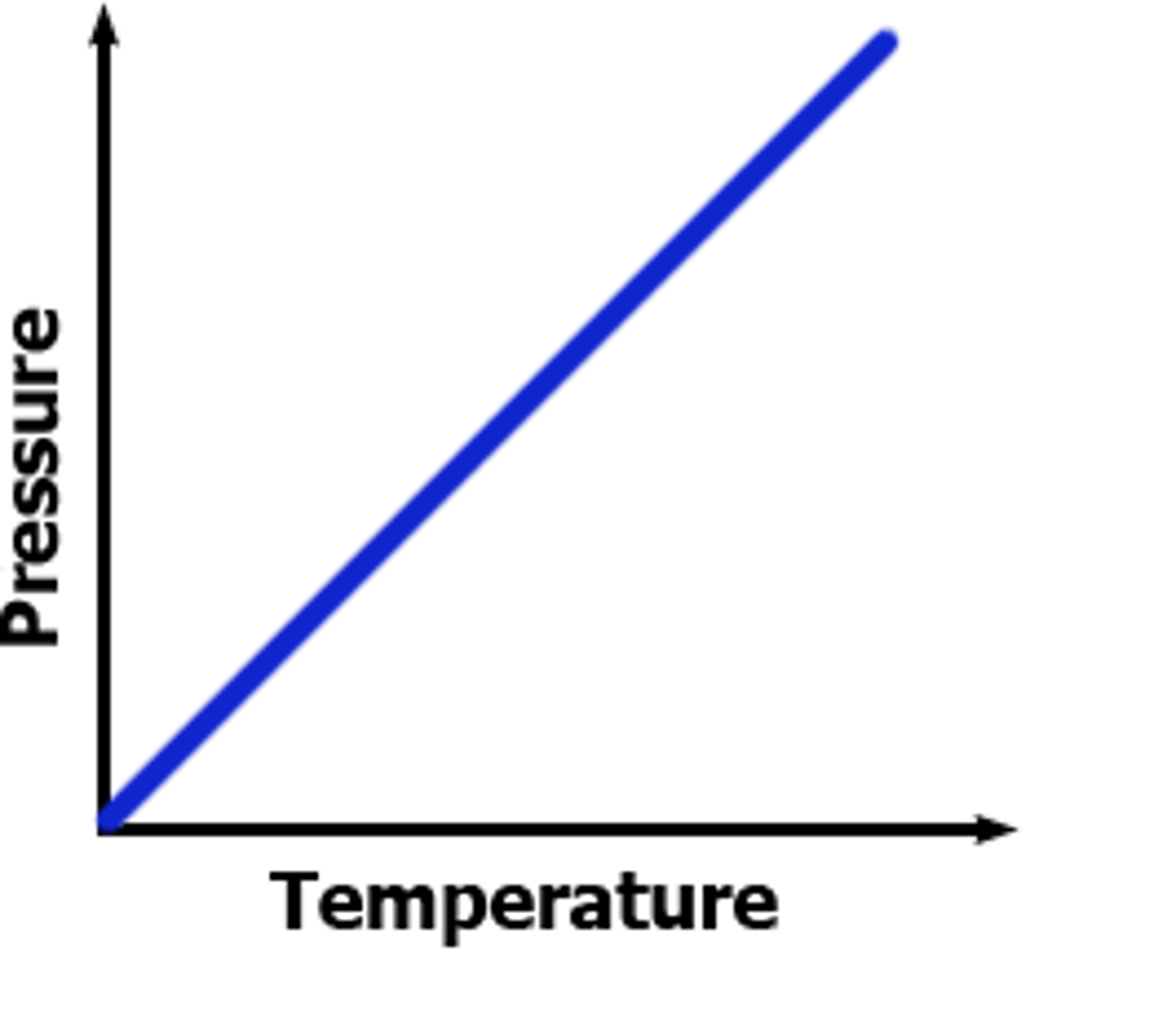
Gay-Lussac's Law Graph
units for pressure
kPa (kilopascal) or atm (atmosphere)
units for volume
mL (milliliter) or L (liter)
units for temperature
C (celsius) or K (kelvin)
Boyle's Law
For a given mass of a gas at constant temperature, volume varies inversely with pressure.
Charles' Law
For a given mass of a gas at constant pressure, volume varies directly with temperature.
Gay-Lussac's Law
For a given mass of gas at constant volume, pressure varies directly with temperature.
Combined Gas Law
This law states that the product of the volume and pressure of an ideal gas divided by its temperature is constant
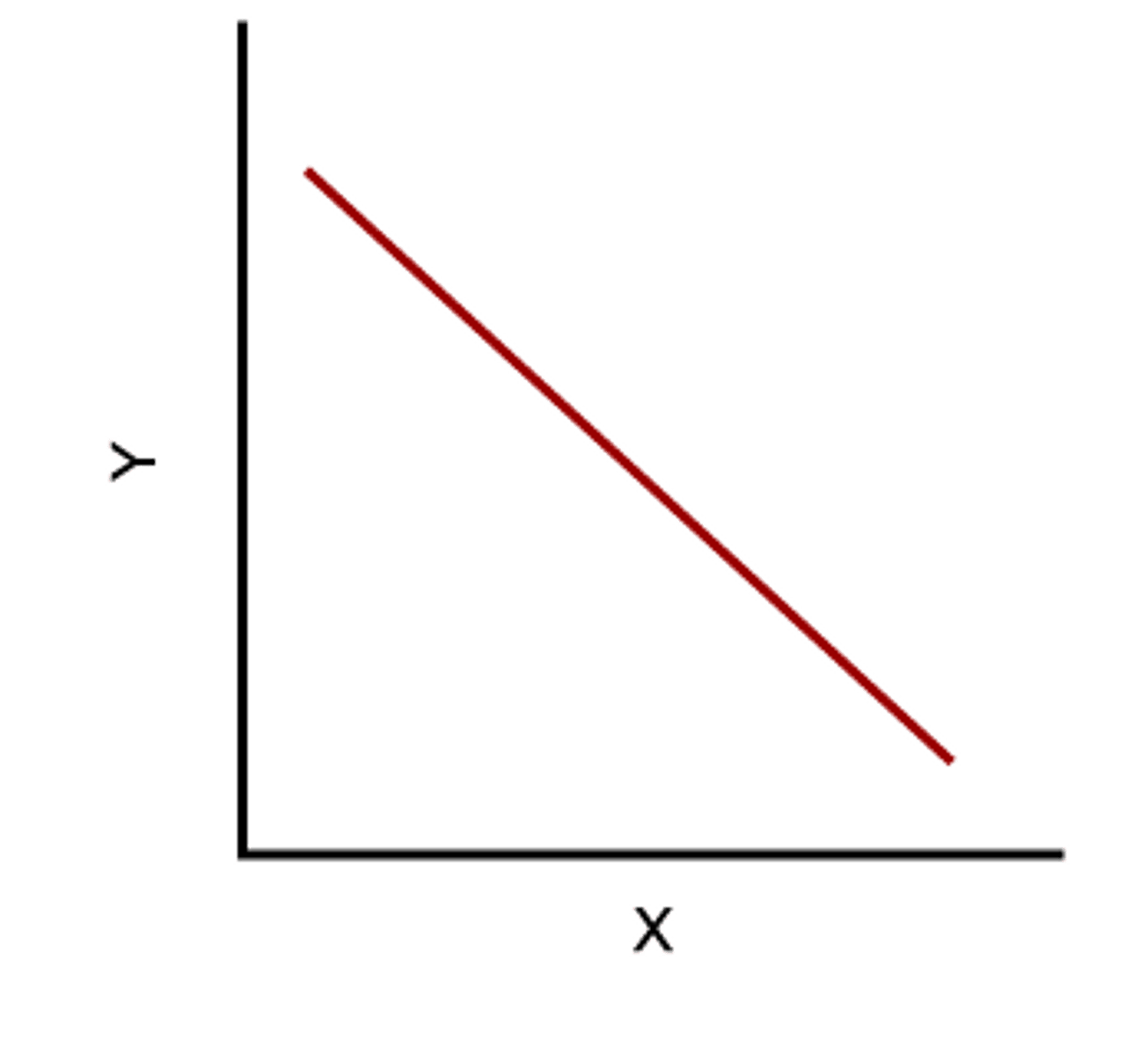
Inverse Relationship
When one goes up, the other goes down.
Direct Relationship
When one goes up, the other goes up.
Boyles