Unit 6.1.4: Oxides reacting with water
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
Period 3 element | Na | Mg | Al | Si | P | S |
|---|---|---|---|---|---|---|
Period 3 oxide | ||||||
Relative melting point | ||||||
Chemical bonding | ||||||
Structure | ||||||
Element |
Complete the table
Period 3 element | Na | Mg | Al | Si | P | S |
|---|---|---|---|---|---|---|
Period 3 oxide | Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO2 |
Relative melting point | High | High | Very high | Very high | Low | Low |
Chemical bonding | Ionic | Ionic | Ionic (with a degree of covalent) | Covalent | Covalent | Covalent |
Structure | Giant ionic | Giant ionic | Giant ionic | Giant covalent | Simple molecular | Simple molecular |
Element | Na | Mg | Al | Si | P | S |
Electronegativity | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 |
Oxide | Chemical equation | pH | Comments |
|---|---|---|---|
Na2O | |||
MgO | |||
Al2O3 | |||
SiO2 | |||
P4O10 | |||
SO2 |
Complete the table for the reactions of oxides with water
Oxide | Chemical equation | pH | Comments |
|---|---|---|---|
Na2O | Na2O (s) + H2O (l) → 2NaOH (aq) | 14 | - |
MgO | MgO (s) + H2O (l) → Mg(OH)2 (aq) | 10 | - |
Al2O3 | No reaction | - | Al2O3 is insoluble in water |
SiO2 | No reaction | - | SiO2 is insoluble in water |
P4O10 | P4O10 (s) + 6H2O (l) → 4H3PO4 (aq) | 2 | Vigorous / violent reaction |
SO2 | SO2 (g) + H2O (l) → H2SO3 (aq) | 1 | - |
Draw a table showing the formula of the oxide, bonding, product with water, pH of product, acid-base reactions and equations of each period 3 element
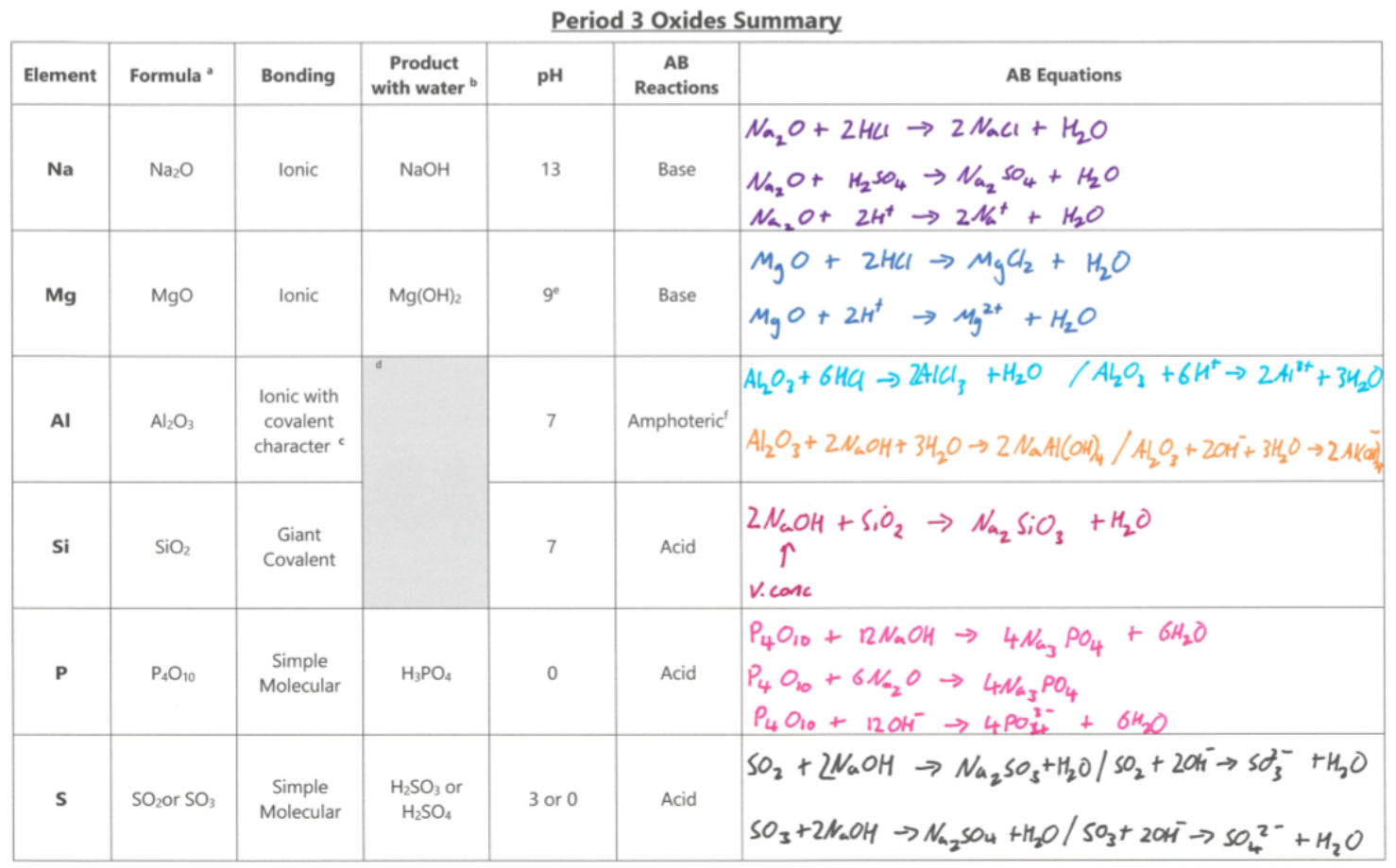
Period 3 oxide | Chemical equation | Comments |
|---|---|---|
Na2O | ||
MgO | ||
Al2O3 | ||
SiO2 | ||
P4O10 | ||
SO2 SO3 |
Complete the acid-base reactions of oxides
Period 3 oxide | Chemical equation | Comments |
|---|---|---|
Na2O | Na2O (s) + 2HCl (aq) → 2NaCl (aq) + H2O (l) | - |
MgO | MgO (s) + 2HCl (aq) → MgCl2 (aq) + H2O (l) | Used in indigestion remedies by neutralising the excess acid in the stomach |
Al2O3 | Al2O3 (s) + 3H2SO4 (aq) → Al2(SO4)3 (aq) + 3H2O (l) | Reacts with acid to form a salt and water |
Al2O3 (s) + 2NaOH (aq) + 3H2O (l) → 2NaAl(OH)4 (aq) | Reacts with hot, concentrated alkali to form a salt | |
SiO2 | SiO2 (s) + 2NaOH (aq) → Na2SiO3 (aq) + H2O (l) | Reacts with hot, concentrated alkali to form a salt and water |
P4O10 | P4O10 (s) + 12NaOH → 4Na3PO4 + 6H2O (l) | - |
SO2 SO3 | SO2 (g) + 2NaOH (aq) → Na2SO3 (aq) + H2O (l) SO3 (g) + 2NaOH (aq) → Na2SO4 (aq) + H2O (l) | - |