Esters
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What is the functional group of esters?
-COO
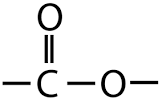
How are esters formed?
They are formed in the reaction between an alcohol and a carboxylic acid in the presence of an acid catalyst
Describe the reaction between ethanol and ethanoic acid in the presence of an acid catalyst
ethanol + ethanoic acid < -acid catalyst- > ethyl ethanoate
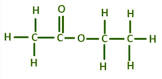
What is the structural formulae of ethyl ethanoate?
CH3COOCH2CH3
What are the rules when writing formulae for an ester?
Remove the -anol from the alcohol and replace it with -yl
Remove the -oic acid from the carboxylic acid and replace it with -oate
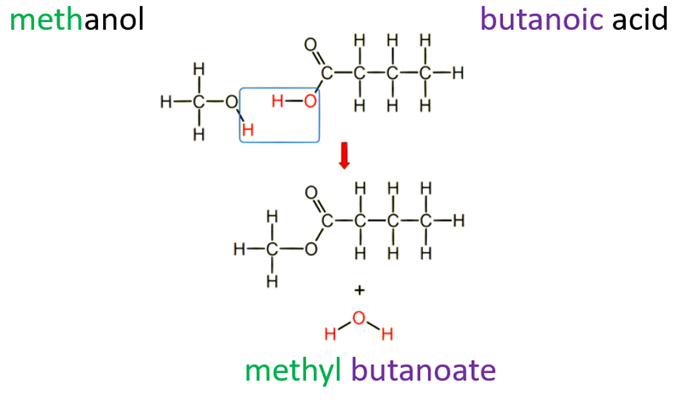
What are the rules for drawing the structural formulae of esters
H is lost from the alcohol
OH is lost from the carboxylic acid
H + OH forms H2O
Bond forms between C from carboxylic acid and O in alcohol
What are esters known and used for?
Esters are known be volatile compounds with distinctive smells
They are used as food flavourings and perfumes
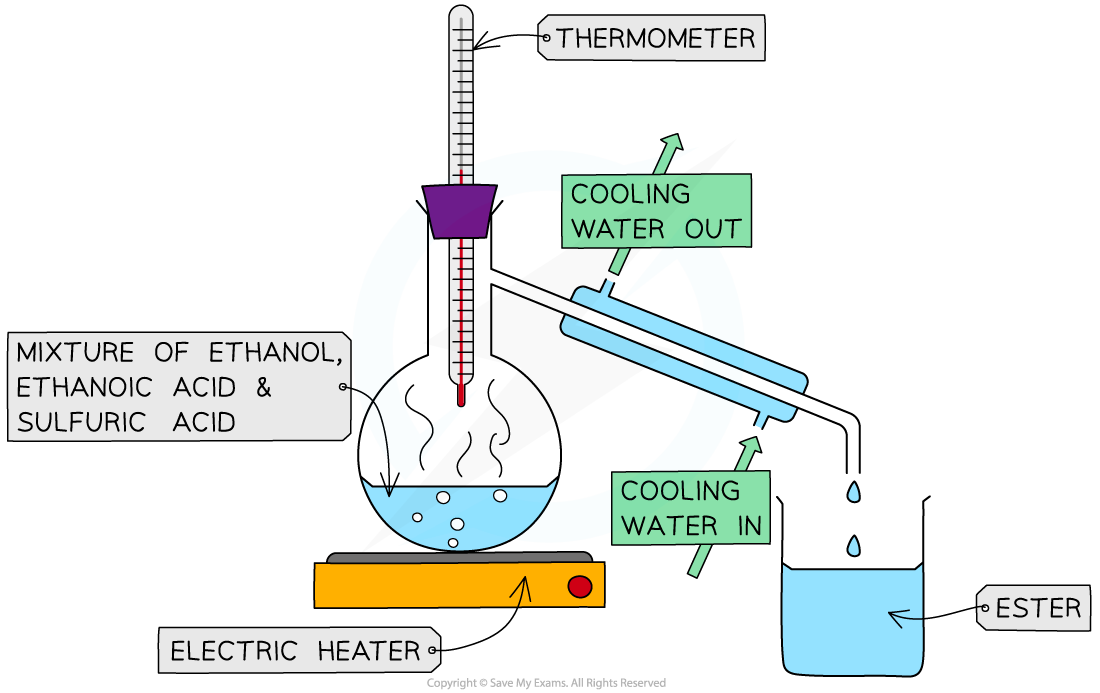
Describe a practical preparing a sample of an ester such as ethyl ethanoate.
A mixture of ethanol, ethanoic acid and concentrated sulfuric acid is heated by a water bath or a electric heater
As esters are volatile, meaning they have low boiling points, they will evaporate first from the mixture
It is then distilled and collected in a separate beaker by condensation through a cooling jacket
The distillate can be smelt to check whether an ester has been produced
Why should we not use a Bunsen burner to heat the mixture?
The mixture contains ethanol, which is flammable
In the case that small quantities of ethanoic acid and sulfuric acid being collected in the process, what can be done to remove them?
To remove acid impurities, add sodium carbonate solution, until the mixture stops fizzing
In the case that ethanol is collected in the process, what can be done to remove it?
Add calcium chloride solution