Hw 2: Alkanes and Cycloalkanes
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
What is the molecular formula of an alkane that has fourteen carbon atoms?
A) C 14 H 28
B) C 14 H 30
C) C 14 H 32
D) C 14 H 34
E) C 14 H 26
B) C 14 H 30
What is the molecular formula of a cycloalkane that has six carbon atoms?
A) C 6 H 12
B) C 6 H 14
C) C 6 H 16
D) C 6 H 10
E) C 6 H 7
A) C 6 H 12
What is the name of the alkane that has four carbon atoms?
A) methane
B) ethane
C) propane
D) butane
E) isobutane
D) butane
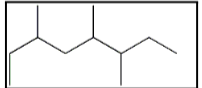
The correct IUPAC name for the following molecule is:
A) 6-ethyl-3,4,-dimethylheptane
B) 2-ethyl-4,5-dimethylheptane
C) 3,4,6-trimethyloctane
D) 3,5,6-trimethyloctane
E) none of these
C) 3,4,6-trimethyloctane
Which of the following molecules does not have only dispersion force?
A) CH 4
B) H 2 O
C) CO 2
D) Cl 2
E) C 2 H 6
B) H 2 O
The name of the alkyl group that contains three carbons is:
A) methyl
B) ethyl
C) propyl
D) isopropyl
E) none of these
C) propyl
Which of the following molecules exhibits hydrogen bonding?
A) CH 4
B) H 2 O
C) CO 2
D) Cl 2
E) H 2 S
B) H 2 O
The name of the alkyl group which contains two carbon atoms :
A) ethyl
B) propyl
C) isopropyl
D) butyl
E) isobutyl
A) ethyl
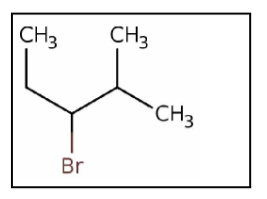
What is the IUPAC name for the following compound?
A) isohexyl bromide
B) 3-bromo-4-methylpentane
C) 1-bromopropylpropane
D) 3-bromo-2-methylpentane
E) 2-methyl-3-bromopentane
D) 3-bromo-2-methylpentane
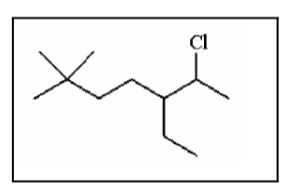
The IUPAC name for the following molecule is:
A) 2-chloro-3-ethyl-6,6-dimethylheptane
B) 6-chloro-5-ethyl-2,2-dimethylheptane
C) 6-chloro-2,2-dimethyl-5-ethylheptane
D) 2,2-dimethyl-5-chloroethylheptane
E) 6-chloro-5-ethyl-2-dimethylheptane
B) 6-chloro-5-ethyl-2,2-dimethylheptane
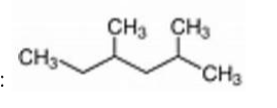
The IUPAC name for the following molecule is:
A) 2-ethyl-4-methylpentane
B) 4-methyl-2-methylpentane
C) 2,4-dimethylhexane
D) 1-isopropyl-2-methylbutane
E) 2,4-methylhexane
C) 2,4-dimethylhexane
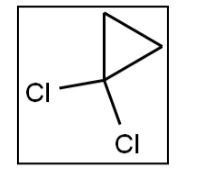
What is a correct name for the following molecule?
A) 2,2-dichlorocyclopropane
B) 1,1-dichlorocyclopentane
C) 1,1-dichloropropane
D) 1,1-dichlorocyclobeutane
E) 1,1-dichlorocyclopropane
E) 1,1-dichlorocyclopropane
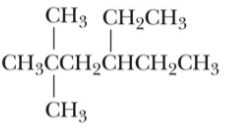
The most stable conformation of propane is:
A) normal hexane
B) 4-ethyl-2,2-dimethylhexane
C) 3-ethyl-5,5-dimethylhexane
D) 4-ethyl-2,2-dimethylhexene
E) 3-ethyl-5,5-dimethylhexyne
B) 4-ethyl-2,2-dimethylhexane
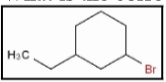
What is the correct name for the following cycloalkane?
A) bromoethylcyclohexane
B) cyclohexane-1,3- bromide
C) 1-bromo-3-ethane
D) cyclohexane
E) 1-bromo-3-ethylcyclohexane
E) 1-bromo-3-ethylcyclohexane
The correct IUPAC name for (CH 3 ) 2 CHCH(CH 3 )(CH 2 ) 3 CH(CH 3 ) 2 is
A) diisopropylpentane.
B) 1,1,2,6,6-pentamethylhexane.
C) 2,5-diisopropylpentane.
D) 2,3,7-trimethyloctane.
E) 1,4-diisopropylpentane.
D) 2,3,7-trimethyloctane.
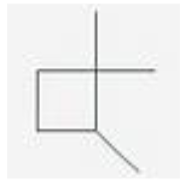
The correct IUPAC name for is:
A) 1,2,2-trimethylcyclobutane.
B) 1,3,3-trimethylcyclobutane.
C) trans-1,3,3-trimethylcyclobutane.
D) 1,1,2-trimethylcyclobutane.
E) 2,2,4-trimethylcyclobutane.
D) 1,1,2-trimethylcyclobutane.
Which intermolecular force is present in all substances by default?
A) Dipole–dipole interactions
B) Hydrogen bonding
C) Ion–dipole interactions
D) London dispersion forces
E) All of them
D) London dispersion forces
Which of the following would exhibit hydrogen bonding?
A) CH 3 Cl
B) CH 3 OH
C) CH 4
D) CH 2 Cl 2
E) CH 3 CH 3
B) CH 3 OH
Which of the following alkanes would have the highest boiling point?
A) pentane
B) 2-methylbutane
C) 2,2-dimethylpropane
D) hexane
E) 2-methylpentane
D) hexane
What statement does NOT apply to the boiling points of alkanes?
A) The boiling point increases as the length of the carbon chain increases.
B) Straight chain alkanes have a higher boiling point than their branched isomers.
C) Because they are nonpolar, alkanes have lower boiling points than other organic
compounds of similar molar mass.
D) The boiling points are affected by Van der Waals attractions.
E) The boiling points are influenced by hydrogen bonding.
E) The boiling points are influenced by hydrogen bonding.
Which cycloalkane has the highest boiling point?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) cyclooctane
E) cyclooctane
The boiling points of normal alkanes
A) rise as the length of the carbon chain increases.
B) rise as the length of the carbon chain decreases.
C) are higher than the boiling points of branched alkanes with the same molecular
formula.
D) a and c
E) b and c
D) a and c
What is the major intermolecular force between molecules of CH₃Cl?
A) London dispersion forces
B) Dipole–dipole interactions
C) Hydrogen bonding
D) Ion–dipole interactions
E) None of them
B) Dipole–dipole interactions
The unusually high boiling point of HF compared to HCl is mainly due to:
A) stronger covalent bonding in HF
B) greater molar mass of HF
C) hydrogen bonding in HF
D) stronger London dispersion forces in HF
E) strong dipole-dipole force
C) hydrogen bonding in HF
1-Bromopropane and 2-bromopropane are
A) resonance
B) homologs
C) isomers
D) conformar
E) All of the above
C) isomers