Module 5: Leaving the Cell
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
72 Terms
What happens to proteins after they are transported into the RER?
Undergo post-translational modifications in the ER lumen
Modifications are essential for folding and function
Include glycosylation, disulphide bond formation, folding, and proteolytic cleavage
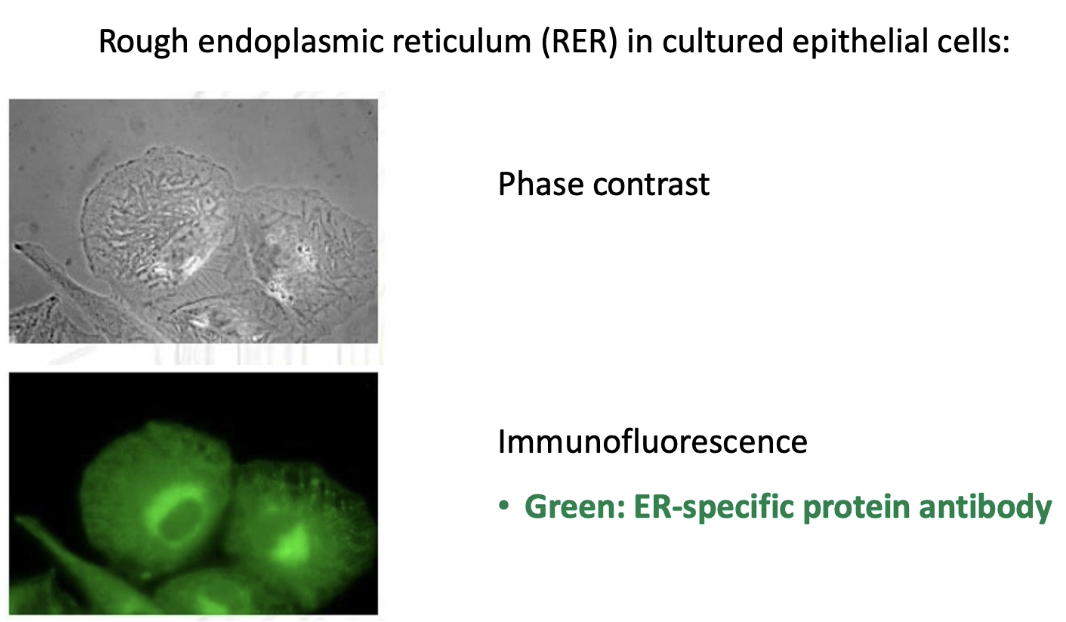
What are the advantages of using phase contrast vs. immunofluorescence microscopy to visualize the ER?
Phase contrast microscopy:
Shows all cell membranes.
Provides greater structural detail.
Immunofluorescence microscopy:
Uses antibody specific to ER protein.
Highlights only the ER membranes.
Allows targeted visualization of ER.
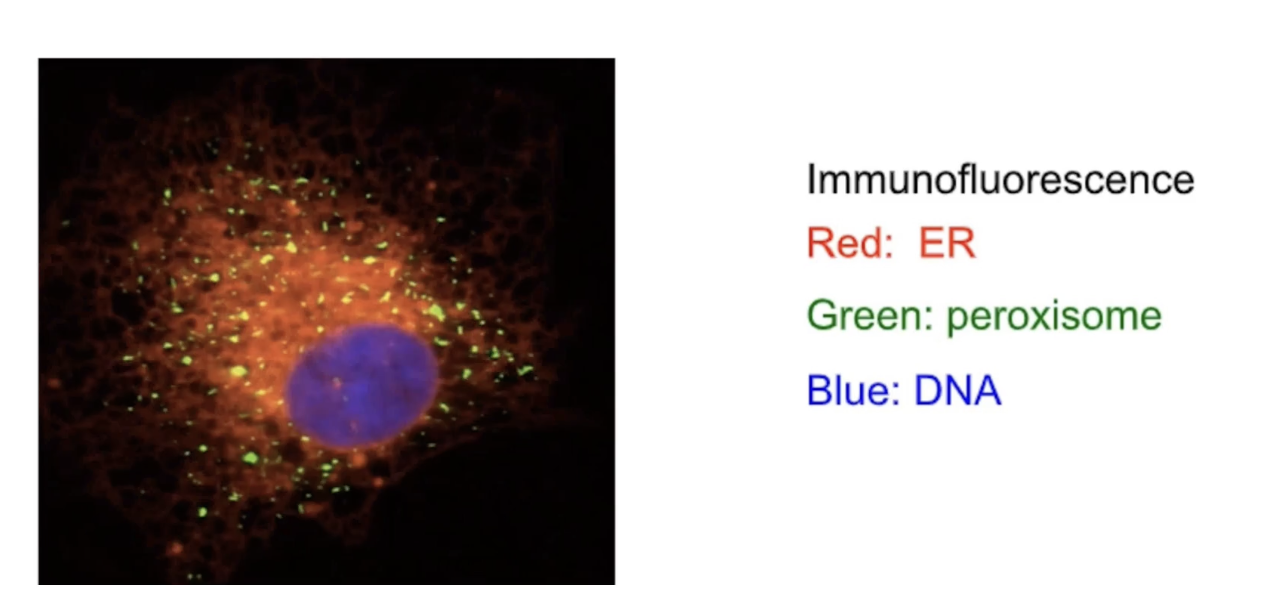
What does live imaging of the ER reveal about its behavior in cells?
ER is a dynamic organelle.
Undergoes constant fission and fusion.
Changes shape and structure.
Migrates to new locations within the cell.
Can be visualized using fluorescent labeling:
DNA (blue)
Peroxisomes (green)
ER (red)
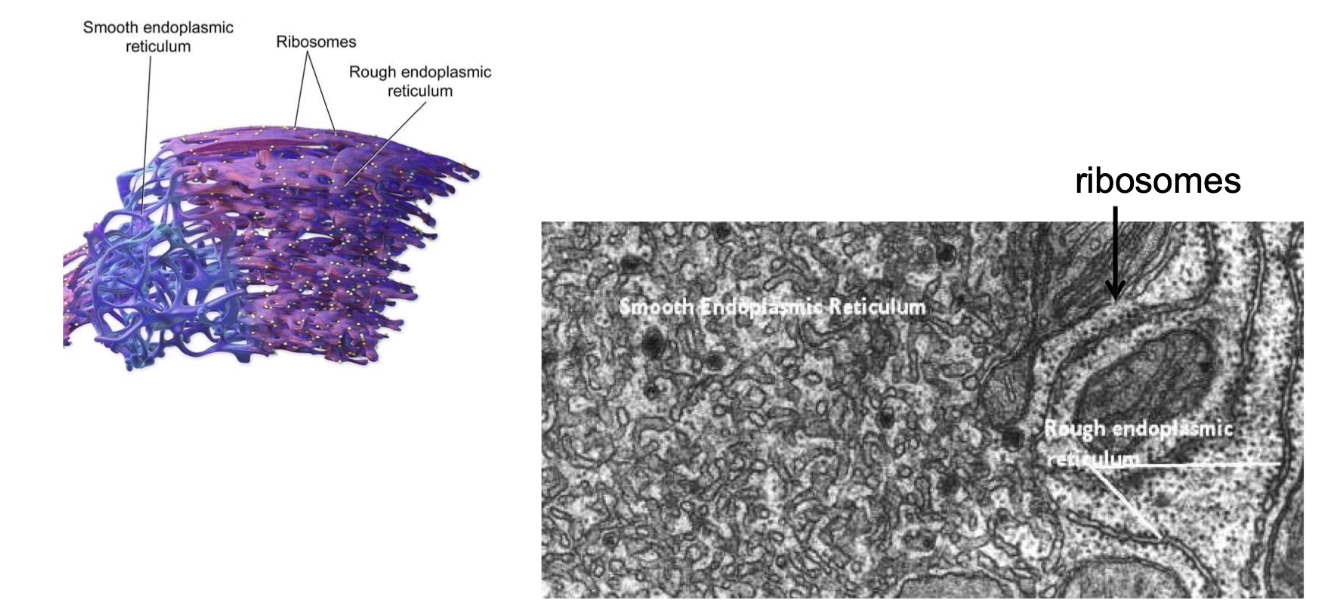
What is the difference between rough ER (RER) and smooth ER (SER) in terms of structure and function?
RER:
Has ribosomes on surface (appears rough seen through EM)
Site of co-translational transport
Protein modification and vesicle formation
SER:
No ribosomes (appears smooth)
Site of lipid synthesis, carbohydrate metabolism, and calcium storage
What types of post-translational modifications occur in the RER?
Glycosylation (addition of carbohydrate groups)
Disulfide bond formation
Protein folding
Proteolytic cleavage (cutting peptide backbone)
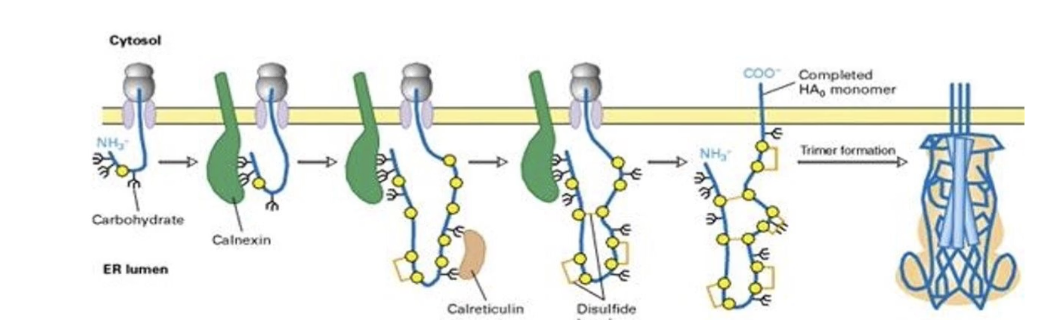
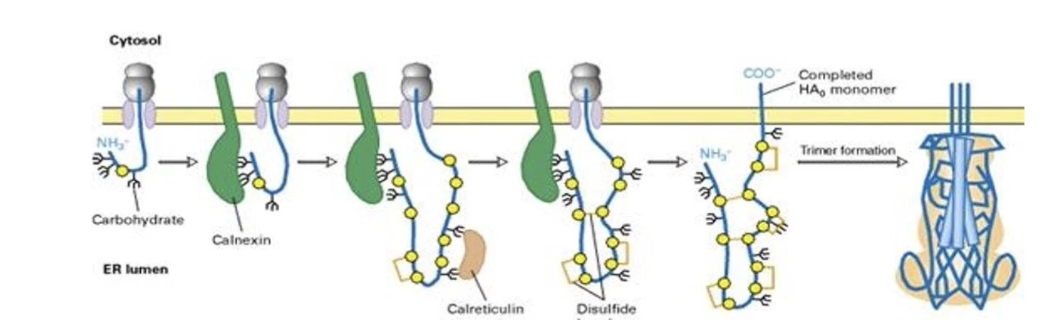
Where do protein modifications occur in RER-targeted proteins?
Soluble (lumenal) proteins: modified along the entire length
Membrane proteins: modifications only on luminal/exoplasmic portions and not the transmembrane/cytoplasmic portion
What is glycosylation and why is it important?
Addition of sugar chains (polysaccharides) to proteins
Important for:
Secretion and membrane insertion
Cell–matrix interactions
Receptor-ligand recognition
Folding, stability, and function
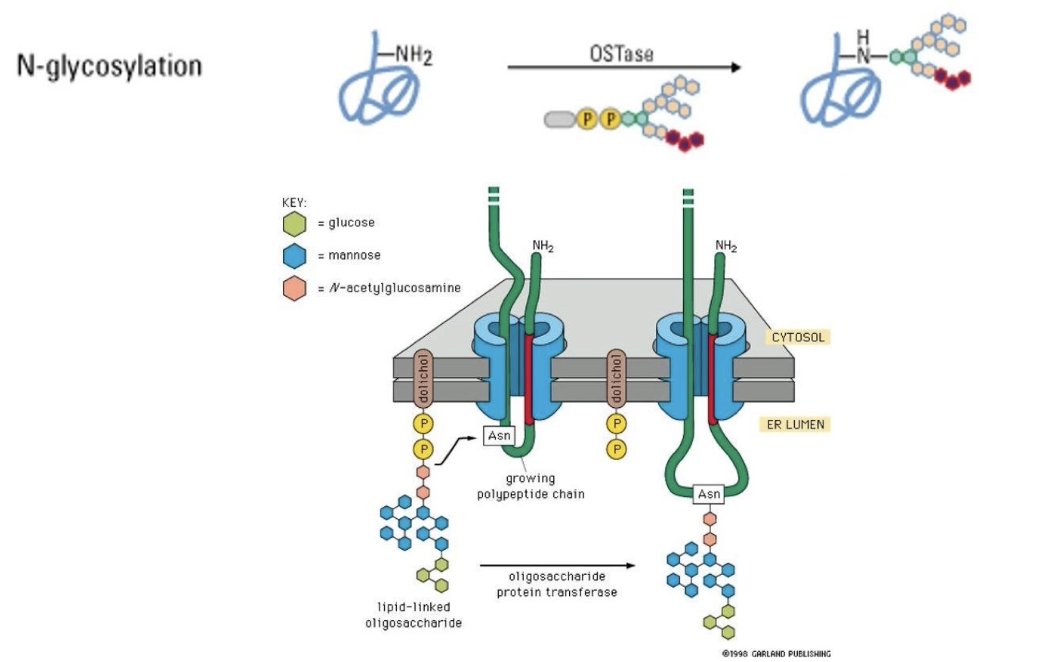
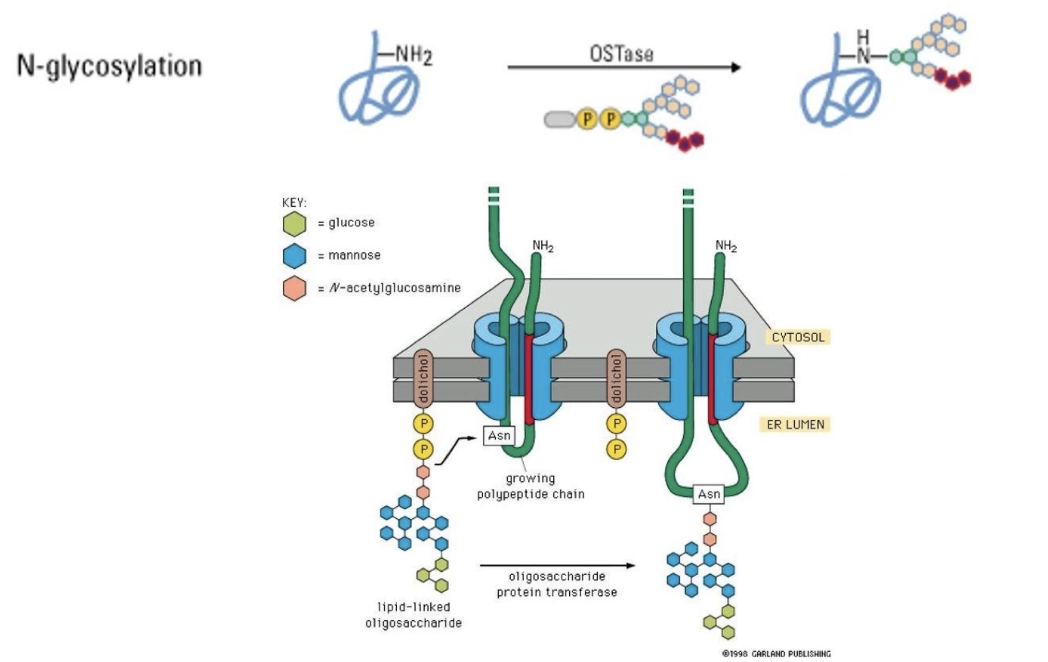
What is N-linked glycosylation?
Sugar group is added to NH₂ of asparagine R-group
Occurs in ER lumen
Modified region remains on luminal/extracellular side
Further modifications can occur within ER and different regions of Golgi
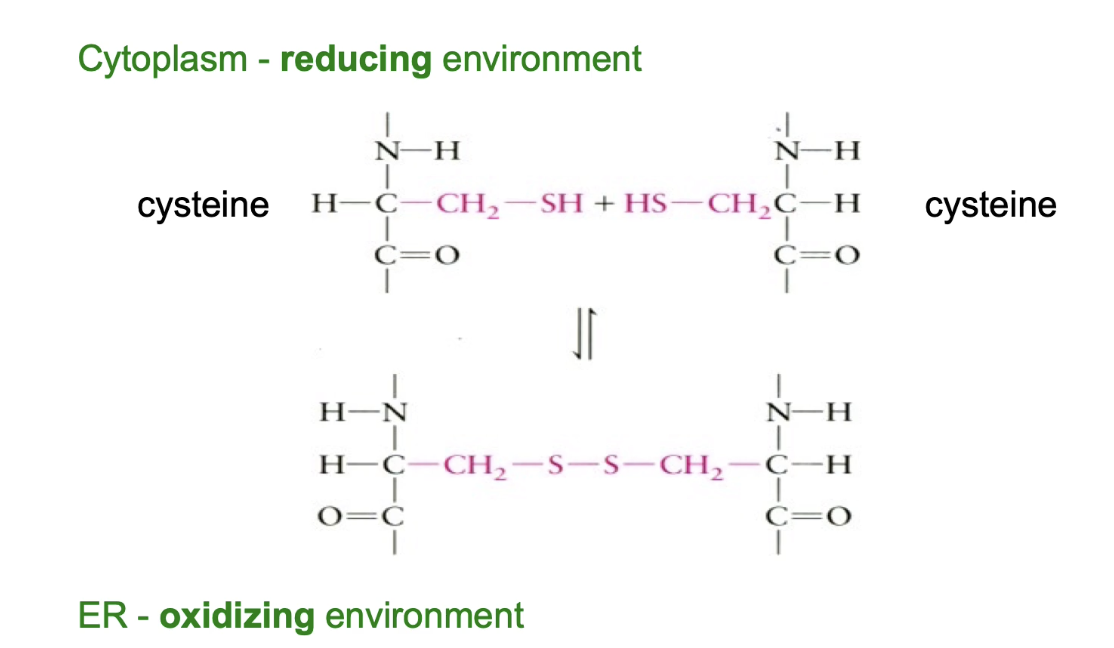
What are disulphide bonds and where do they form in eukaryotic cells?
Covalent bonds between two -SH groups of cysteine residues.
Help stabilize tertiary or quaternary protein structure.
Can be:
Intramolecular (within one protein).
Intermolecular (between two proteins).
Form in the ER lumen due to:
Oxidizing environment favoring bond formation.
Cytoplasm has a reducing environment that reverses disulphide bonds.
Common in:
Secreted proteins.
Cell surface proteins.
Provide stability against harsh, denaturing conditions outside the cell.
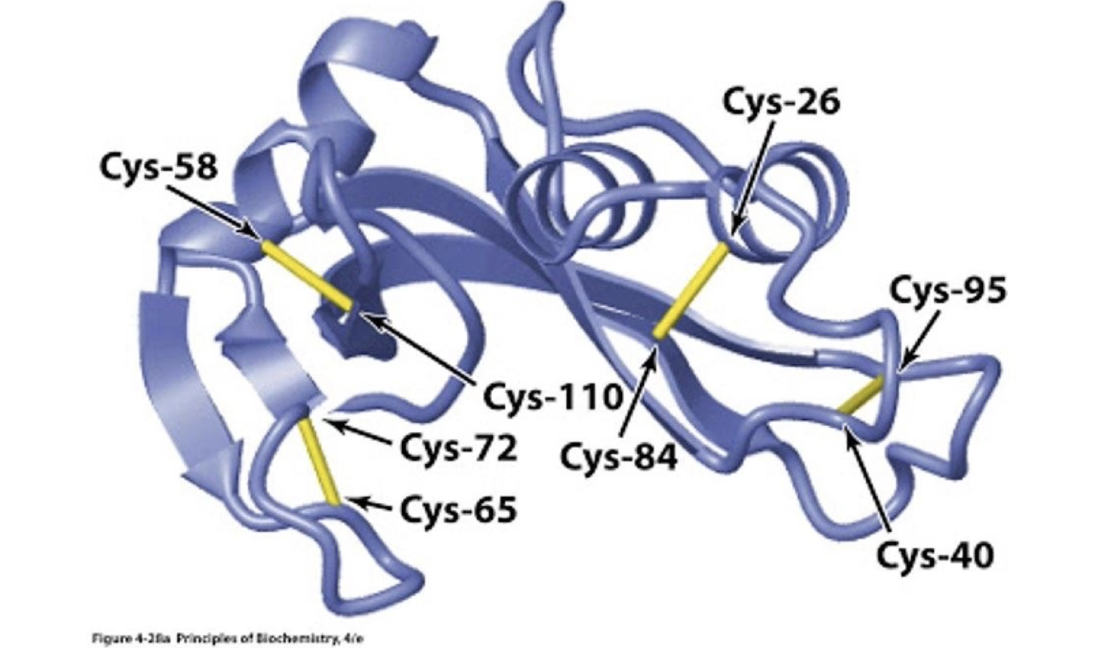
What is an example of a protein with disulphide bridges, and what is their
Pancreatic ribonuclease A (RNAse A).
Contains four disulphide bridges.
Secreted into the intestine.
Function: digests RNA by cleaving it into smaller pieces.
Disulphide bonds:
Help maintain structure in acidic intestinal conditions.
Preserve the enzyme's functional state.
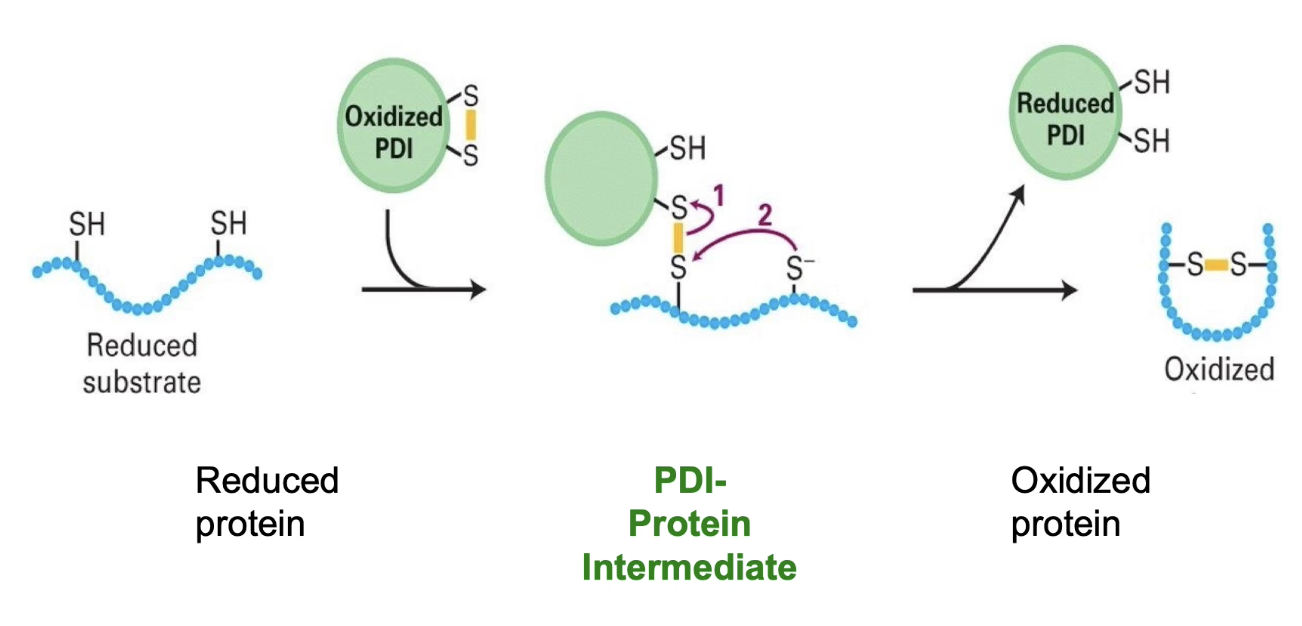
What is Protein Disulphide Isomerase (PDI) and what does it do?
PDI is oxidized in the ER lumen (contains a disulphide bond itself).
PDI interacts with a substrate protein containing cysteine residues.
Forms an intermediate by temporarily bonding with a cysteine residue in the substrate.
Transfers its disulphide bond to the substrate, forming an intramolecular disulphide bridge.
PDI becomes reduced during the process.
PDI is re-oxidized spontaneously in the ER’s oxidizing environment.
PDI can also rearrange incorrect disulphide bonds (acts as an isomerase to correct folding errors).
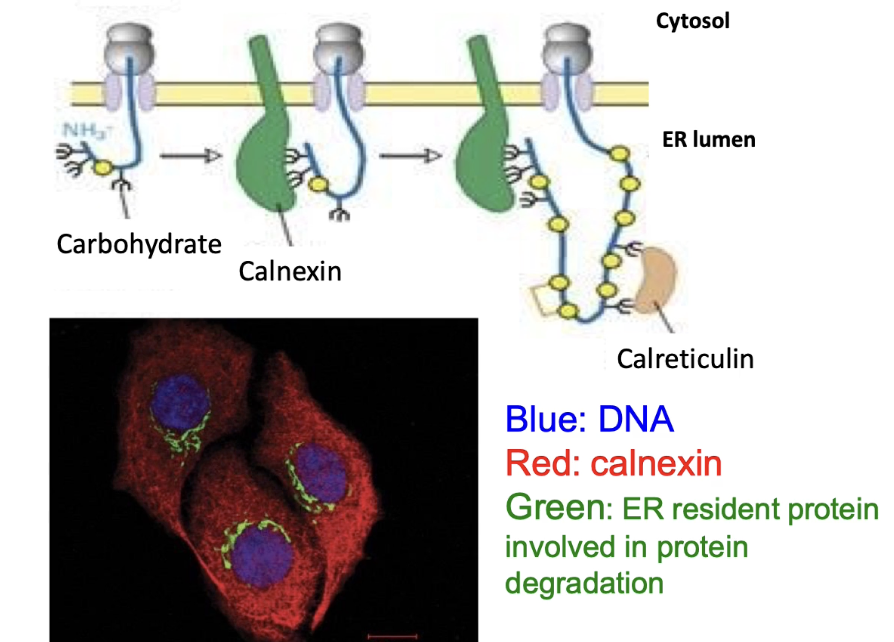
What are lectins and how do they help protein folding?
Proteins that bind glycosylated proteins
Act like molecular chaperones
Examples: Calnexin (found throughout ER membrane) and Calreticulin
Help proper folding of glycosylated proteins in ER
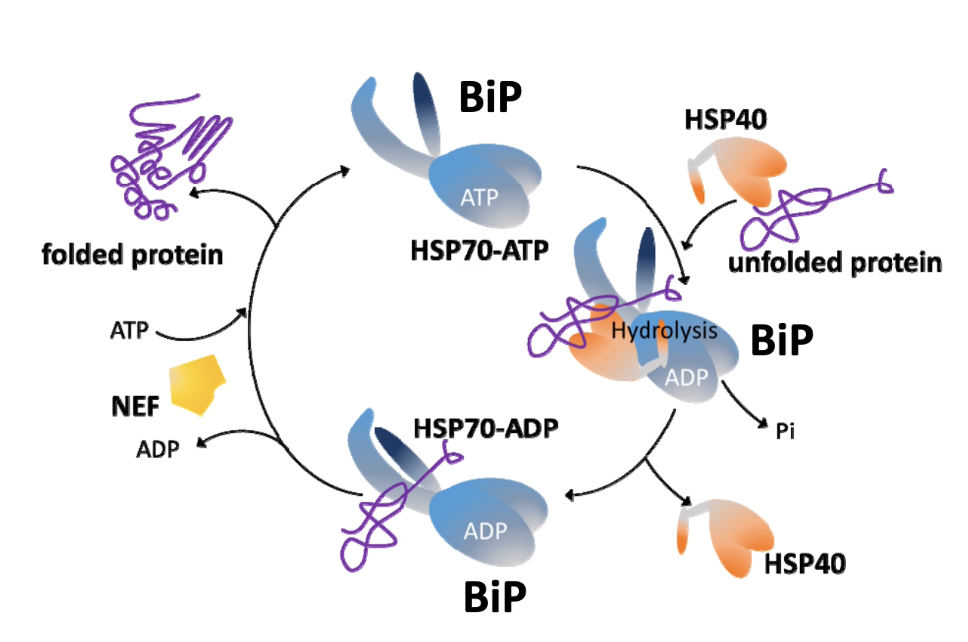
What is BiP and what are its functions?
BiP (Binding immunoglobulin Protein) is a member of the HSP70 family and resides in the ER.
Binds to unfolded proteins.
Roles of BiP:
Assists in co-translational protein transfer into the ER:
Binds to nascent polypeptides emerging into the ER lumen via the translocon.
Works with co-chaperones:
Hsp40 helps in substrate recognition.
NEF (Nucleotide Exchange Factor) promotes ADP release and ATP binding for cycling activity.
Ensures proper folding of proteins inside the ER.
Plays a central role in initiating the Unfolded Protein Response (UPR) during ER stress.
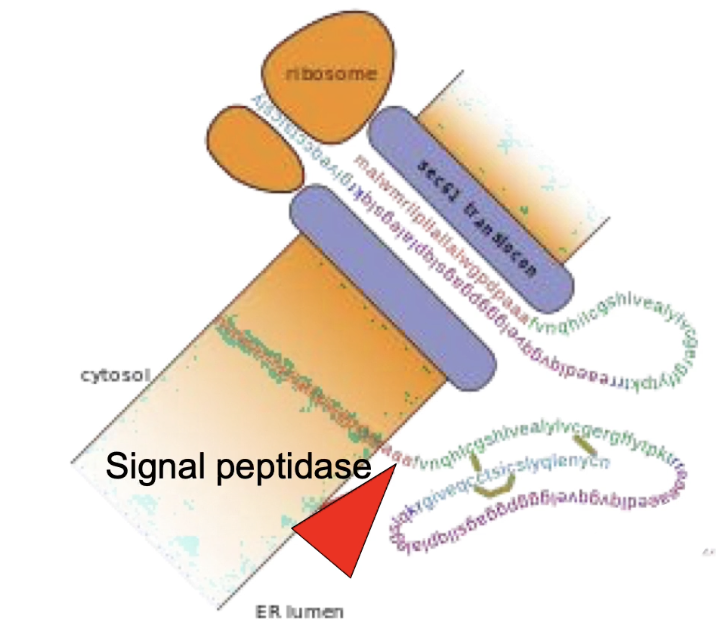
What is proteolytic cleavage and what is an example?
Proteolytic cleavage = cutting of the peptide backbone of a protein.
Occurs within the ER lumen as a post-translational modification.
Purpose: Required for proper protein folding and function.
Example:
Type I integral membrane proteins:
N-terminal signal sequence is cleaved by signal peptidase.
Proinsulin (precursor of insulin):
Signal peptidase removes N-terminus in the ER.
Disulphide bridges stabilize the folded structure.
Further processed by three additional peptidases during transport through secretory vesicles in pancreatic cells.
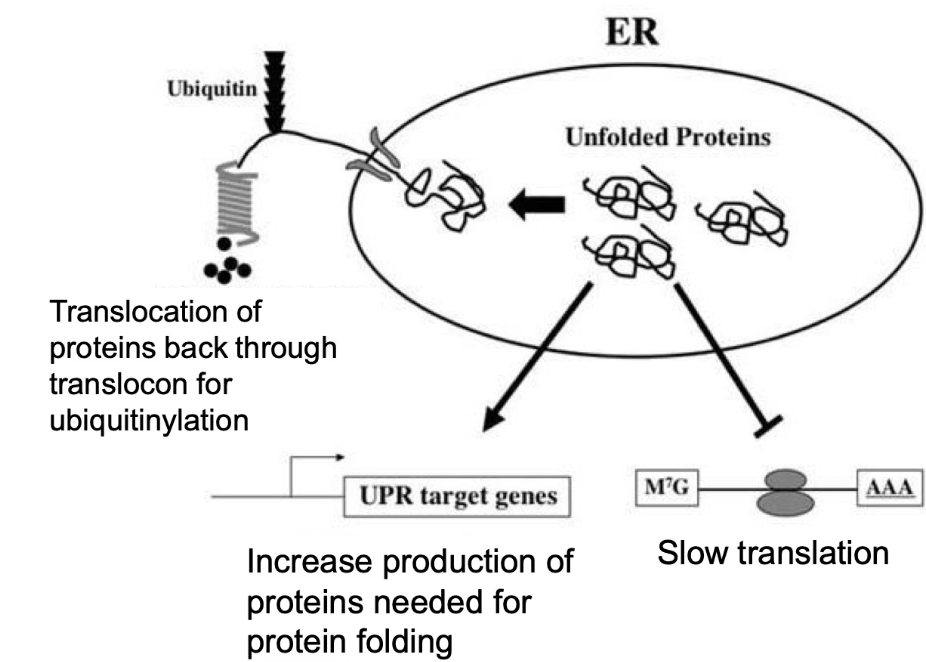
What is the Unfolded Protein Response (UPR) and when is it triggered?
Triggered by:
Accumulation of unfolded proteins in the rough ER.
Caused by overproduction, stress (heat/toxins), or nutrient lack.
Why it's important:
Unfolded proteins can't exit ER → risk of aggregation and cell death.
Steps of UPR:
Slow down new protein translation.
Degrade unfolded proteins (via ubiquitinylation).
Increase chaperone proteins to assist folding.
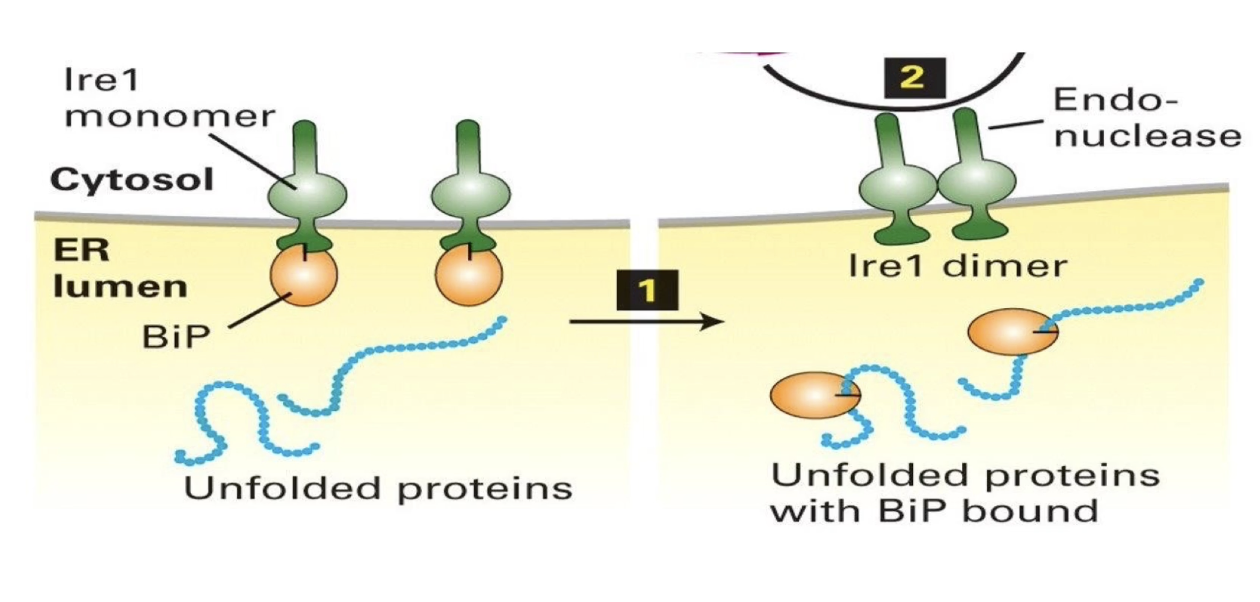
How do BiP and Ire1 function in the Unfolded Protein Response (UPR)?
UPR = cellular response to detect and manage unfolded/misfolded proteins in the ER
Goal: Give proteins time/tools to fold properly
BiP (Binding immunoglobulin Protein):
Acts as a chaperone → helps protein folding, prevents aggregation
Inhibits Ire1 when bound to it
Has higher affinity for hydrophobic patches of unfolded proteins, dissociates from Ire1 when misfolded proteins accumulate
Ire1 (Inositol-requiring enzyme 1):
Transmembrane sensor protein in ER
Inactive when bound to BiP
Activates upon BiP dissociation → forms homodimers
Becomes an endonuclease that targets Hac1 mRNA
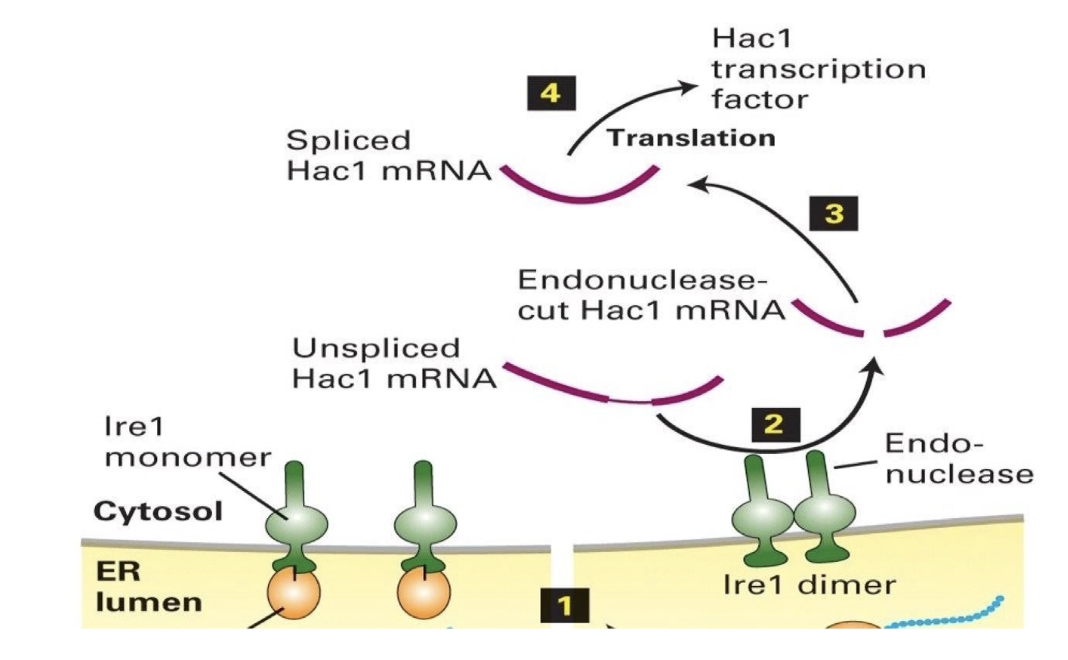
What role does Ire1 play in regulating Hac1 mRNA and protein production during the UPR?
Ire1 splices Hac1 mRNA in the cytosol (not nucleus)
Unspliced Hac1 mRNA contains an inhibitory intron-like sequence that blocks translation
Splicing by Ire1 removes this block, allowing translation of Hac1 protein
Hac1 protein:
Functions as a transcription factor
Enters the nucleus
Activates genes for protein-folding regulators:
BiP
Lectins
PDI (Protein Disulfide Isomerase)
Signal peptidases
Result:
ER enhances its folding capacity to handle misfolded protein load
Elegant feedback loop: misfolded proteins → trigger UPR → produce more folding helpers
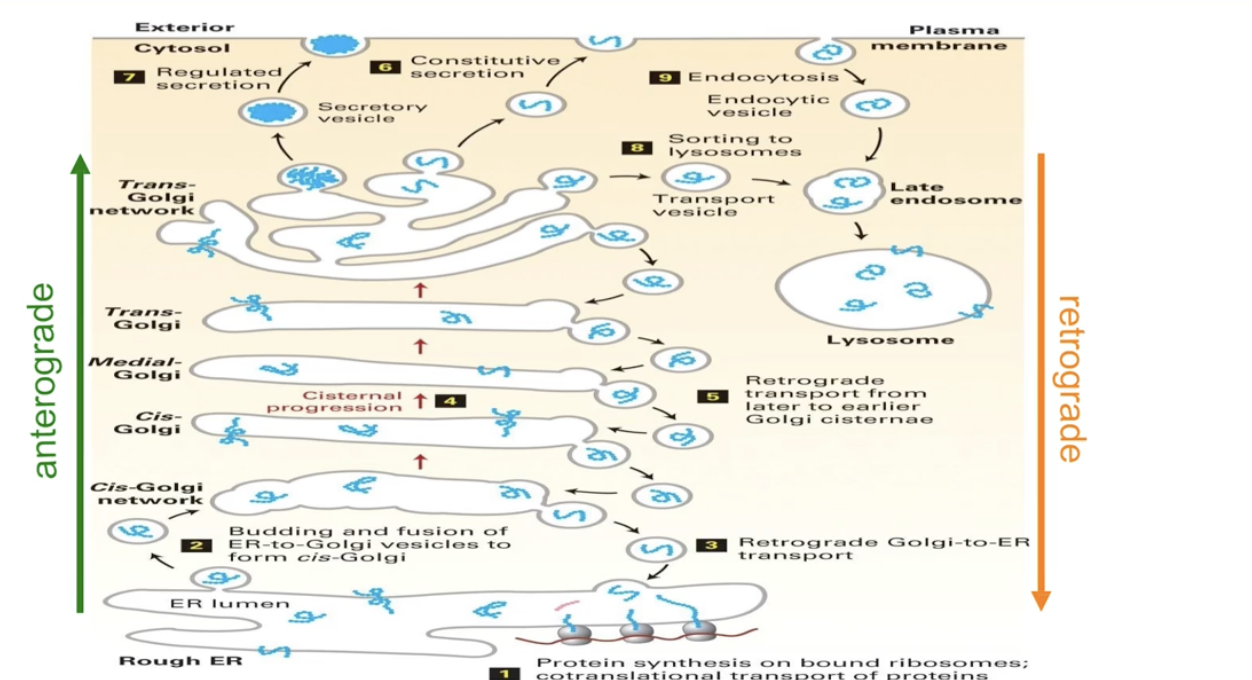
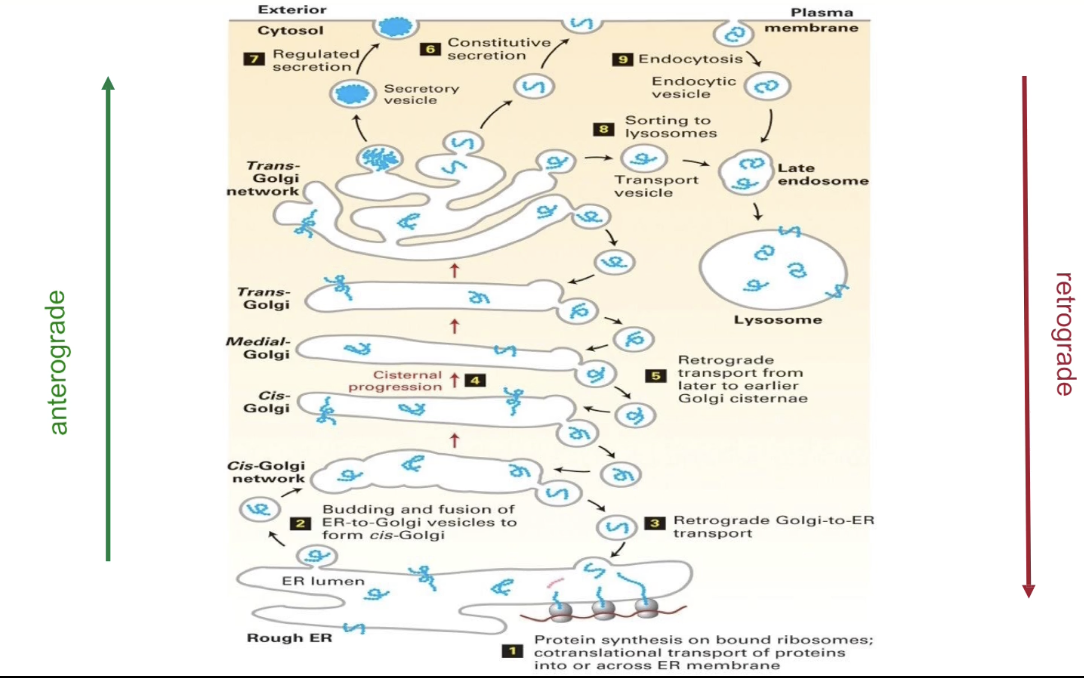
What is the pathway of proteins from the ER to the cell membrane?
Co-translational transport inserts proteins into/across ER membrane
ER-resident proteins stay in the ER
Others leave ER via vesicles to:
Golgi
Lysosome
Cell membrane
Secretion
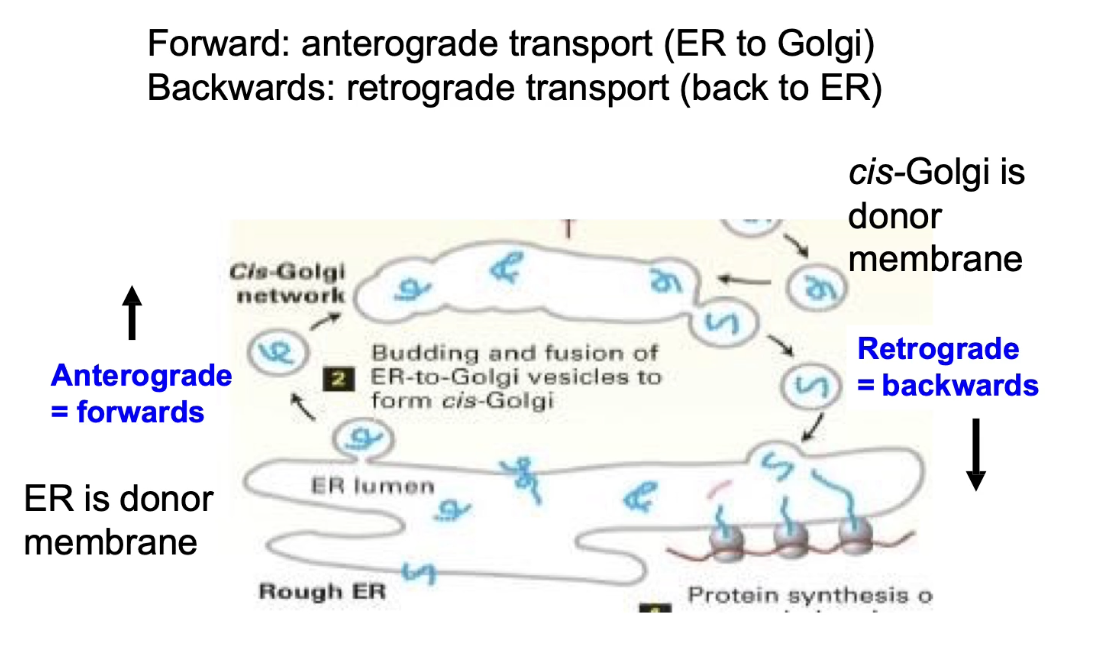
Anterograde transport = ER → Golgi → Cell Membrane
Retrograde transport = returns proteins to ER
How was the protein transport pathway from the ER discovered and studied?
Pulse-chase labeling + immuno-TEM:
First technique to track protein movement in mammalian cells
Fluorescent microscopy with GFP-tagged proteins:
Visualized live protein transport
Yeast mutants with transport defects:
Helped identify key proteins in the pathway
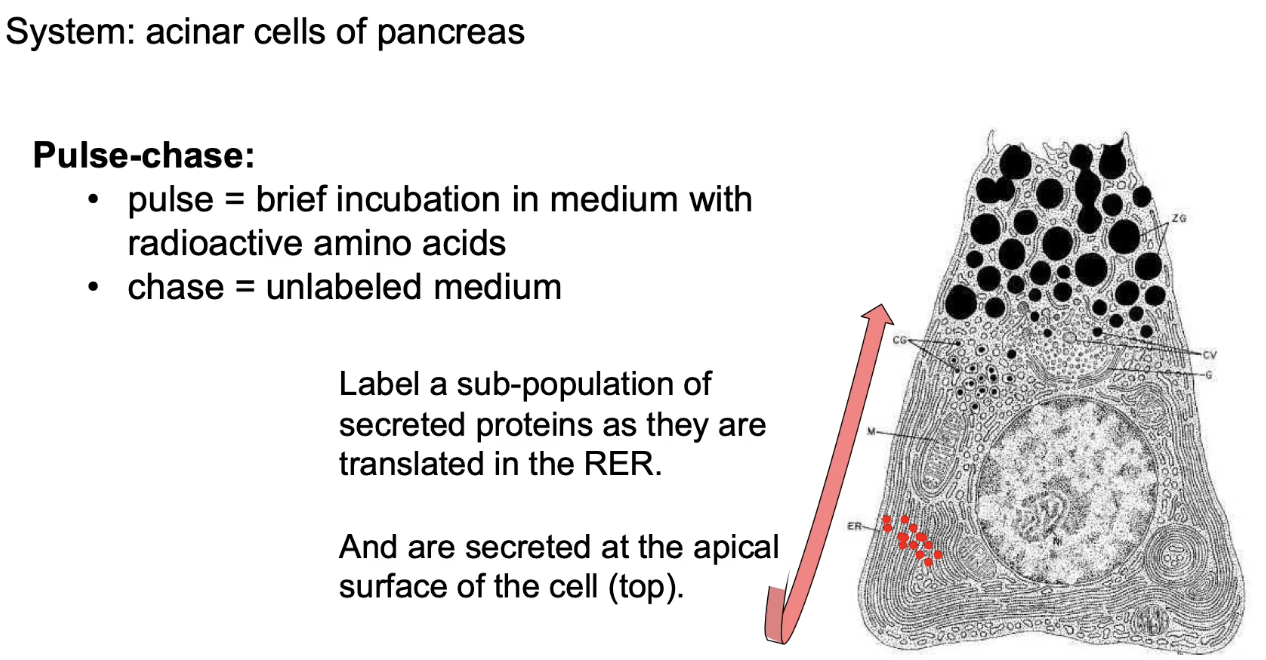
What is a pulse-chase experiment and how is it used to study protein movement?
Used in acinar cells (pancreas) that secrete digestive enzymes
Pulse:
Short exposure (e.g., 3 min) to radioactive methionine
Labels only newly synthesized proteins in the rough ER
Chase:
Cells transferred to medium with non-radioactive amino acids
Allows tracking of labeled proteins over time
Helps visualize protein movement from ER → Golgi → secretion point
What do different time points in a pulse-chase experiment reveal?
0-minute chase: Proteins remain in ER
17-minute chase: Proteins are in Golgi
117-minute chase: Proteins are in secretory vesicles
Tracks progression of proteins through secretory pathway
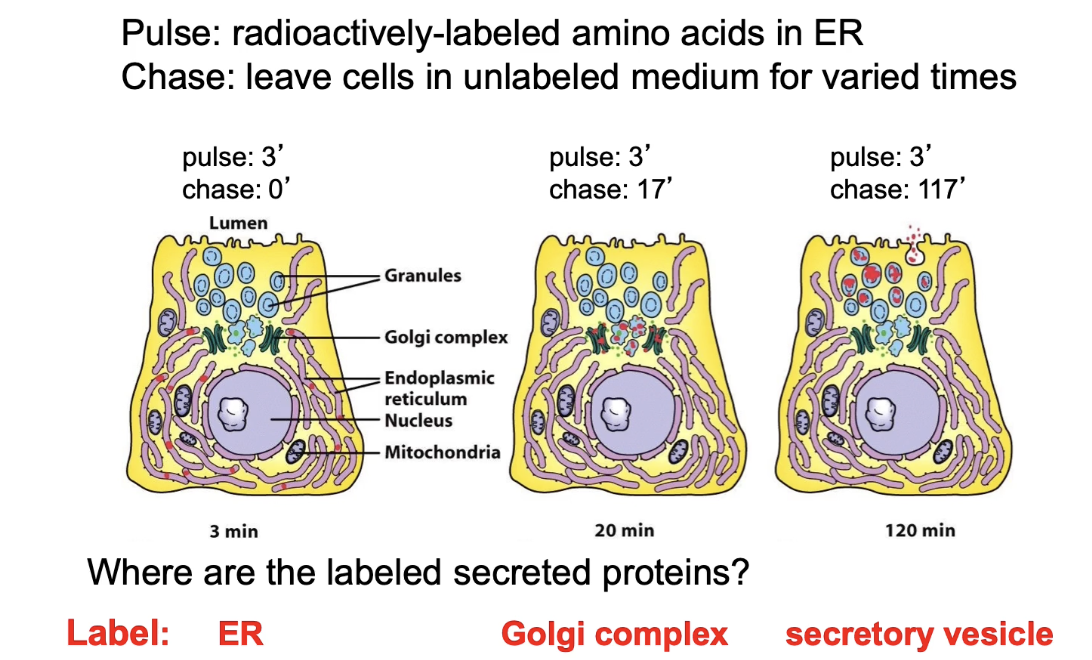
What did the first pulse-chase experiments demonstrate?
Confirmed sequence of protein transport:
RER → Golgi → Secretory vesicles → Cell membrane
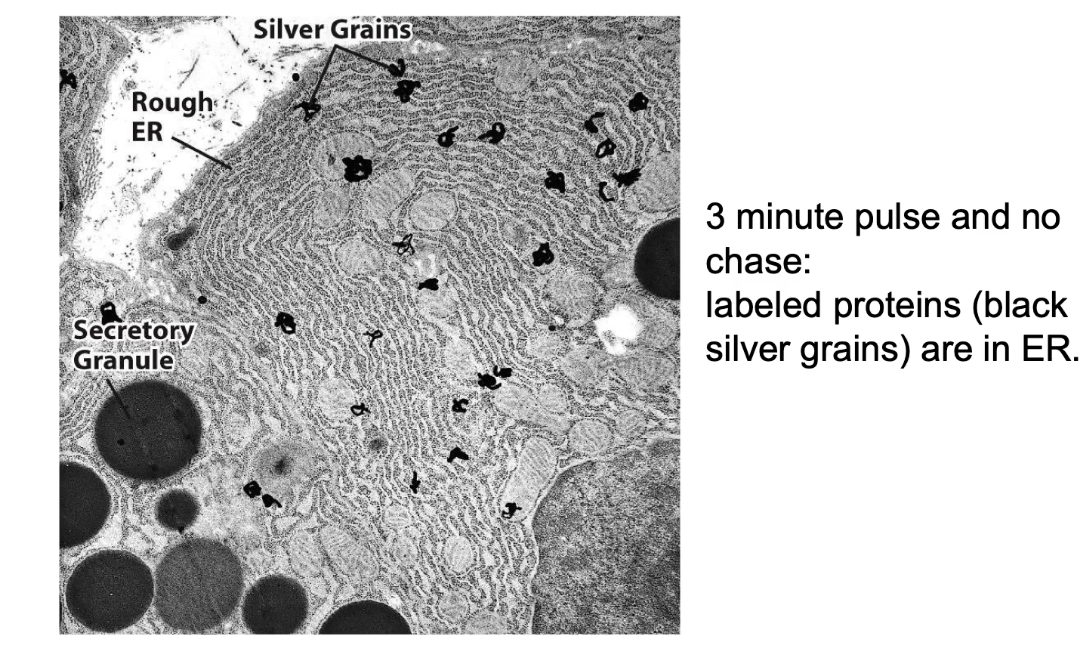
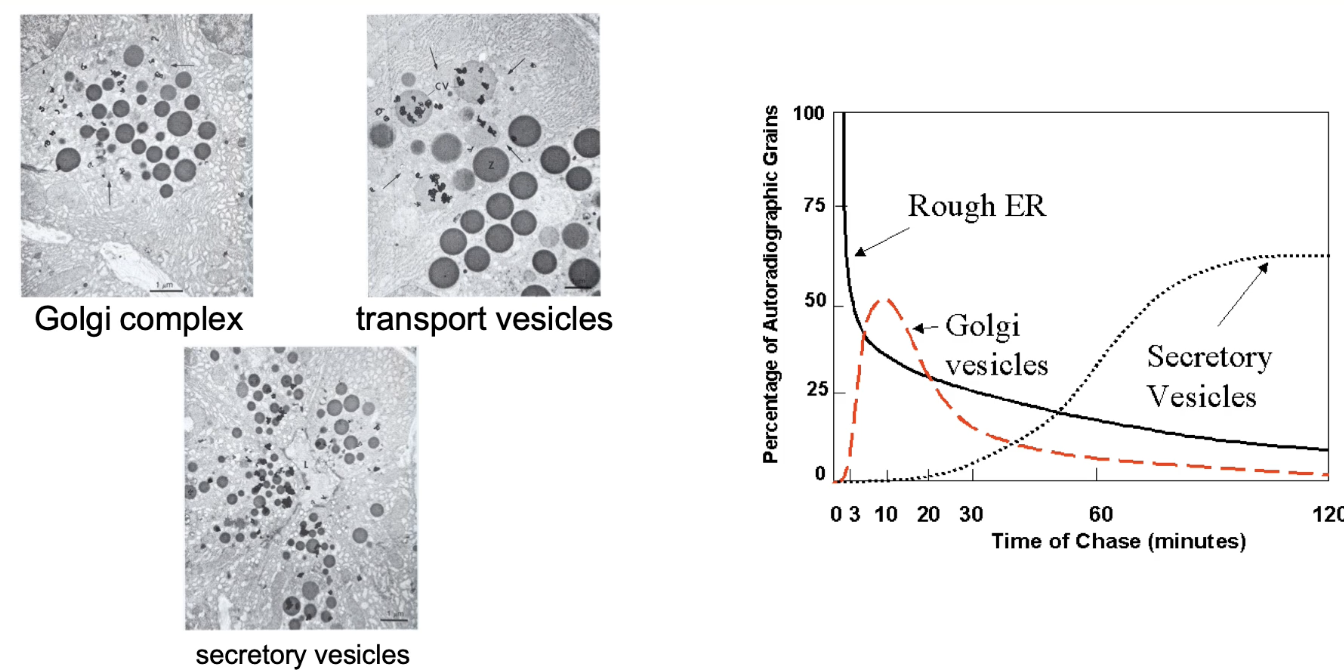
How did transmission electron microscopy (TEM) contribute to understanding the secretory pathway?
TEM provides high-resolution images of cell structures (e.g., ER, Golgi, vesicles)
Radioactively-labeled proteins appear as dark spots ("grains") on the images
Tracking grains over time shows protein movement through secretory pathway
Graph summary:
Y-axis: % of labeled protein (grains) at each location
X-axis: Time (chase duration, 0–120 min)
Shows dynamic shift in protein localization over time
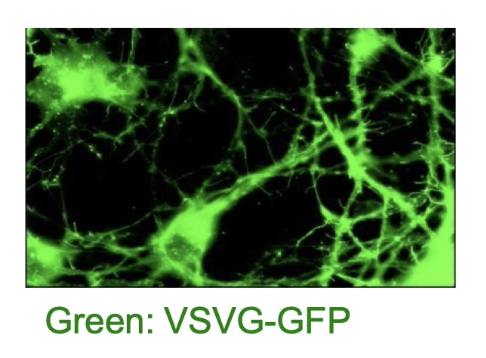
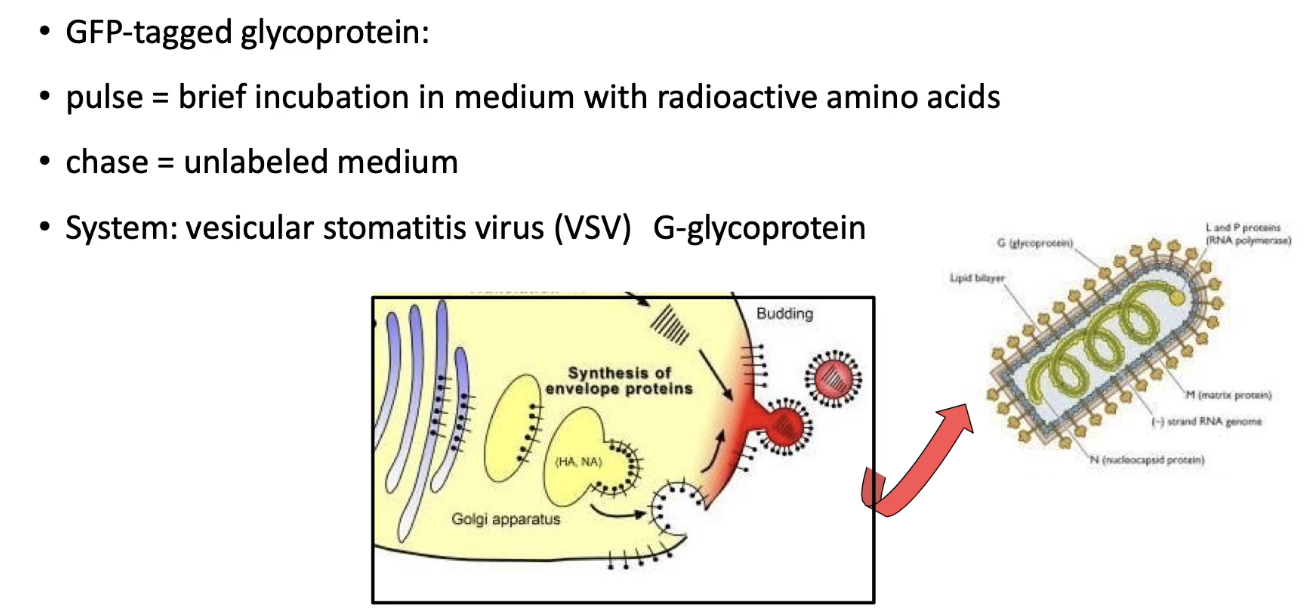
How is the VSV-G protein used to study protein transport in mammalian cells?
VSV virus encodes G-protein, a membrane protein
G-protein is synthesized in host ER, then glycosylated and sent to the cell membrane
Tagged with GFP → VSV-G:GFP allows live-cell tracking via fluorescence
Mutant VSV-G behaves differently at different temperatures:
32°C (permissive): folds properly → transported to membrane
40°C (restrictive): misfolds → retained in ER via UPR
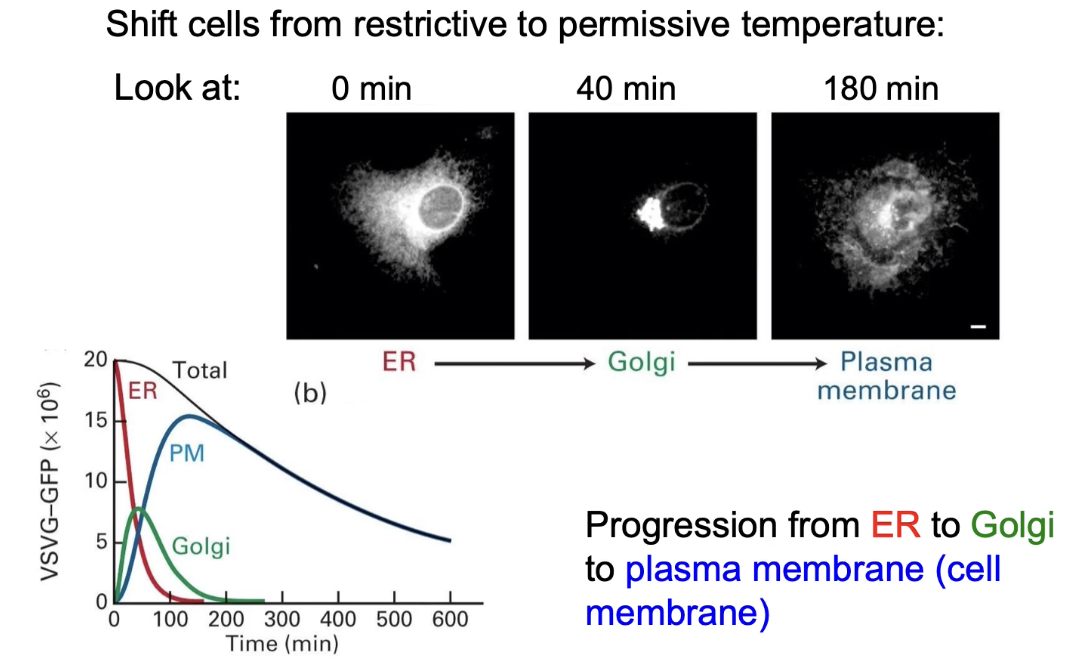
How does temperature shifting help track VSV-G:GFP protein transport?
Cells infected briefly to express VSV-G:GFP
At 40°C: protein misfolds → trapped in ER
Lowered to 32°C: protein folds → moves through secretory pathway
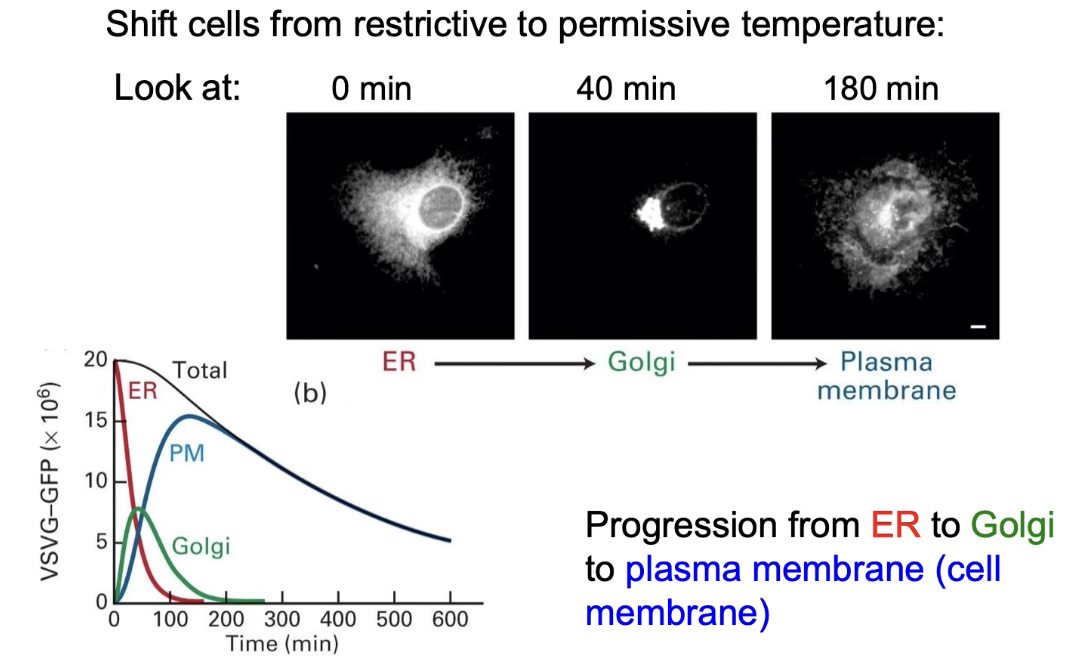
What does fluorescence imaging reveal about VSV-G:GFP movement over time?
0 min (40°C): fluorescence in ER
40 min (32°C): fluorescence in Golgi
180 min (32°C): fluorescence at cell membrane
Graph:
X-axis: time (0–600 min)
Y-axis: fluorescence intensity
Shows protein moving ER → Golgi → membrane
Total fluorescence decreases over time due to fluorophores lose their fluorescence
How did researchers use yeast and invertase to study protein transport pathways?
Used yeast (S. cerevisiae) as a model system
Yeast secretes invertase to hydrolyze sucrose → glucose + fructose
Invertase is secreted via the secretory pathway
Glucose/fructose from one cell can feed neighboring cells
By tracking invertase secretion, researchers can identify defects in protein transport
Simple and effective way to study secretory pathway mutations
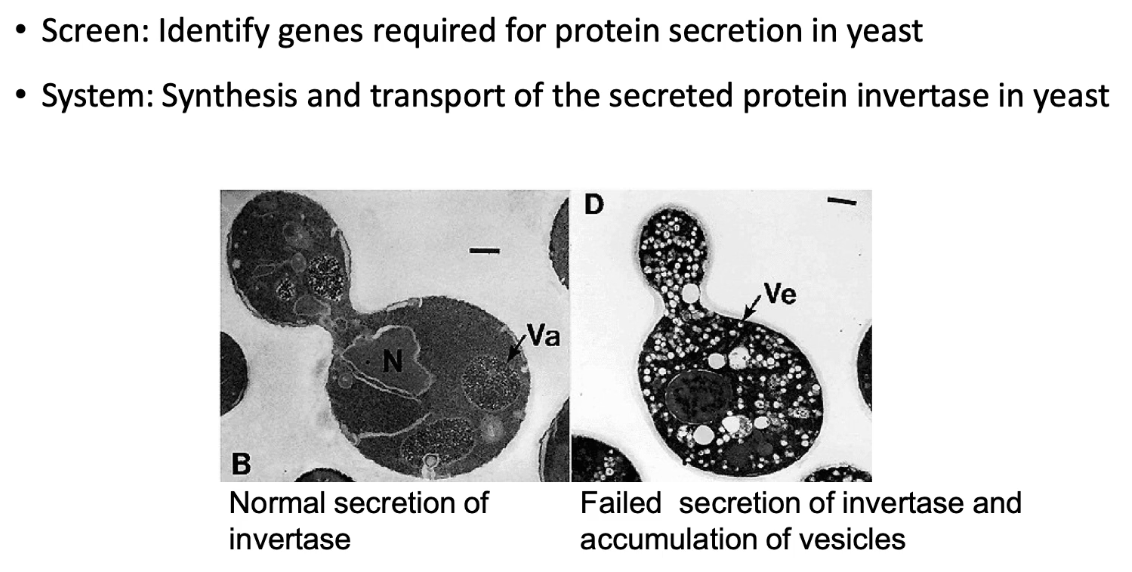
What experimental strategy was used to identify protein transport steps in yeast?
Researchers (e.g., Randy Schekman) induced random mutations in yeast genome
Looked for temperature-sensitive mutants that block invertase secretion
At permissive temp: mutant proteins fold/function normally
At restrictive temp: proteins misfold, causing secretion defect
Mutants where invertase accumulated in vesicles were identified
Each mutant named as a sec (secretory) mutant (e.g., Sec61)
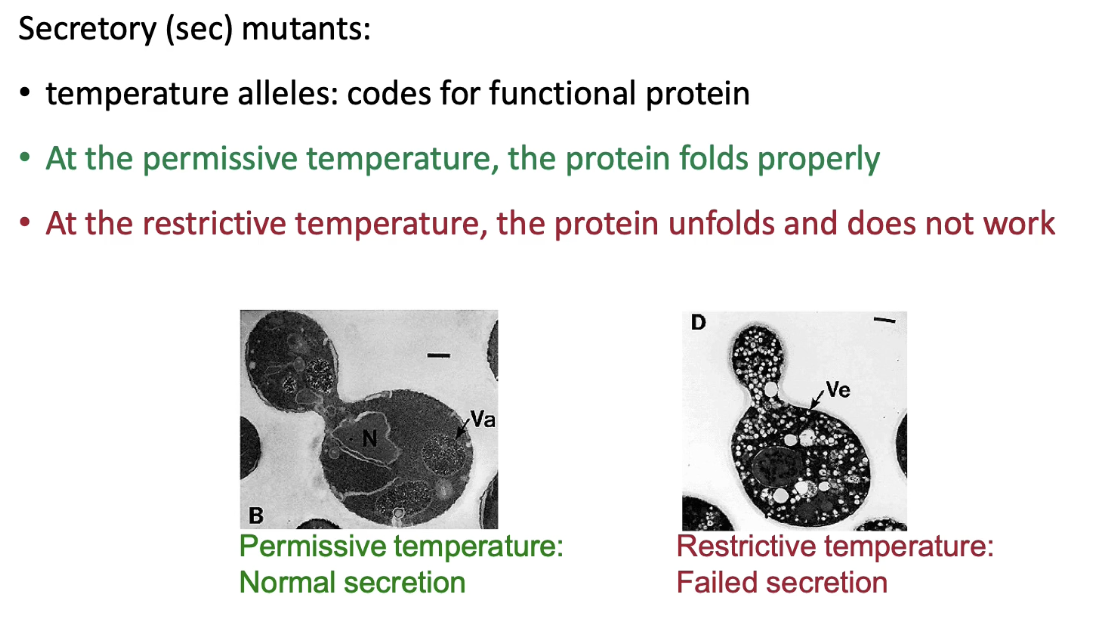
What does a Class A sec mutant indicate in yeast protein transport?
Invertase accumulates in the cytosol
Indicates defect in co-translational transport into ER
Caused by mutations in:
ER translocon (e.g., Sec61)
Signal recognition particle (SRP)
SRP receptor
Signal sequence of invertase
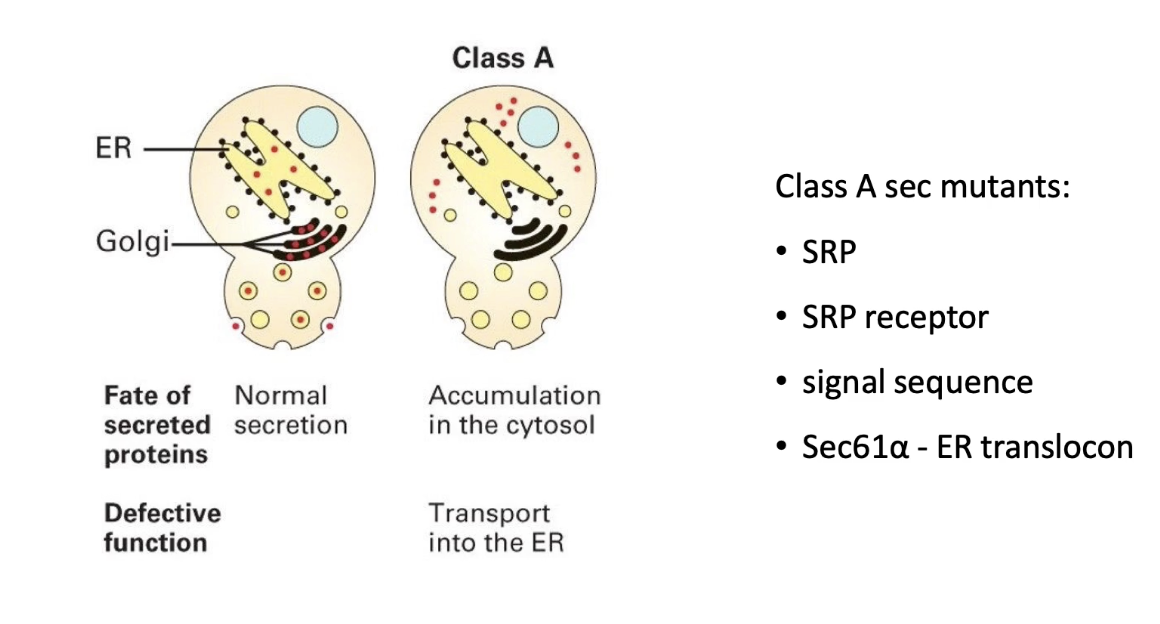
What are the five classes of sec mutants and their associated defects?
Class A: Protein stuck in cytosol (defective ER entry).
Class B: Protein stuck in ER (defective ER exit in vesicle transport).
Class C: Protein stuck in ER-to-Golgi vesicles (defective vesicle fusion with Golgi).
Class D: Protein stuck in Golgi (defective exit from Golgi in vesicle formation from Golgi).
Class E: Protein stuck in secretory vesicles (defective vesicle fusion with plasma membrane).
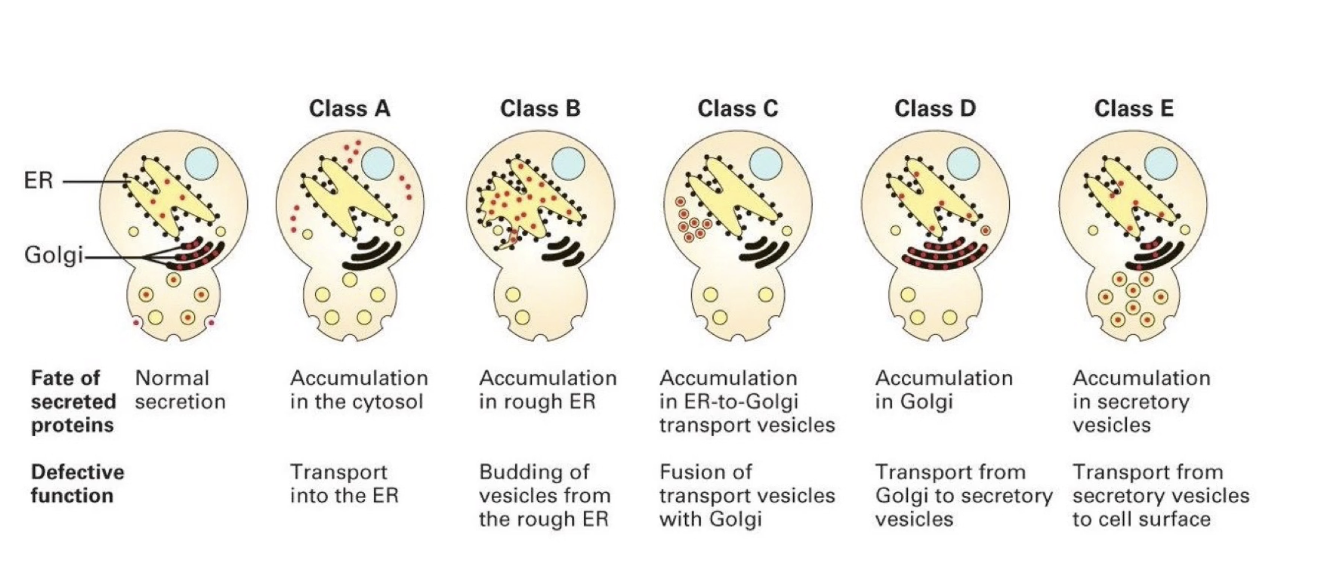
What did double mutant analysis reveal about protein transport order?
Upstream mutations mask downstream phenotypes
Example: Class A + Class B = Class A phenotype
Invertase gets trapped in cytosol, never reaches ER
Confirmed stepwise pathway:
Rough ER → Golgi → Secretory vesicles → Cell membrane
How did yeast sec mutant studies contribute to understanding protein transport?
Identified genes/proteins required at each transport step
Allowed mapping of transport pathway using invertase accumulation
Enabled functional characterization of transport proteins over time
What is the structure and role of the Golgi complex in protein transport?
Made of elongated, flat sacs called cisternae
Vesicles carry proteins:
From rough ER to cis-Golgi cisternae
From trans-Golgi cisternae to final destinations
Site of protein processing and sorting
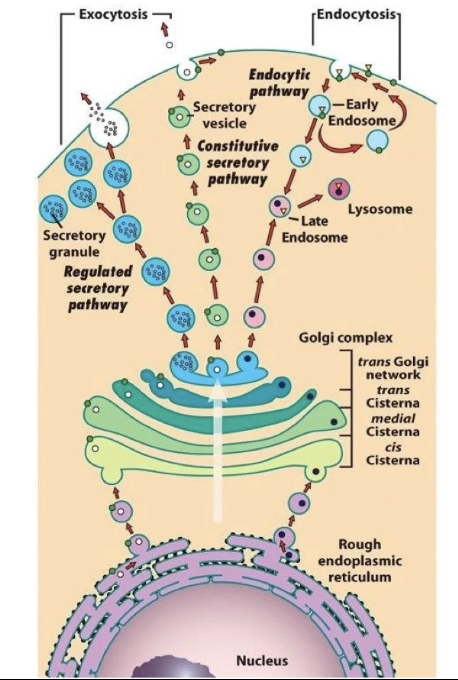
What is exocytosis and how is it related to vesicle transport?
Exocytosis: fusion of vesicles with the cell membrane
Releases proteins out of the cell
Involves vesicles from the trans-Golgi network
What is the difference between the constitutive and regulated secretory pathways?
Constitutive pathway:
Used by proteins that are:
Released immediately after synthesis and transport
Vesicles move directly from trans-Golgi to cell membrane
Constant and not signal-dependent
Regulated pathway:
Proteins are stored in the cell until a signal triggers release
Stored in specialized vesicles called secretory granules
Triggered release (e.g., hormones, neurotransmitters)
How are proteins from the Golgi complex involved in lysosome formation?
Some proteins from the trans-Golgi cisternae are packaged into vesicles.
These vesicles contain enzymes destined for the lysosome.
They fuse with endosomes, which form at the cell membrane and bring in external macromolecules.
Fusion of Golgi vesicles and endosomes forms lysosomes.
This allows internal enzymes to break down materials taken in from outside the cell.
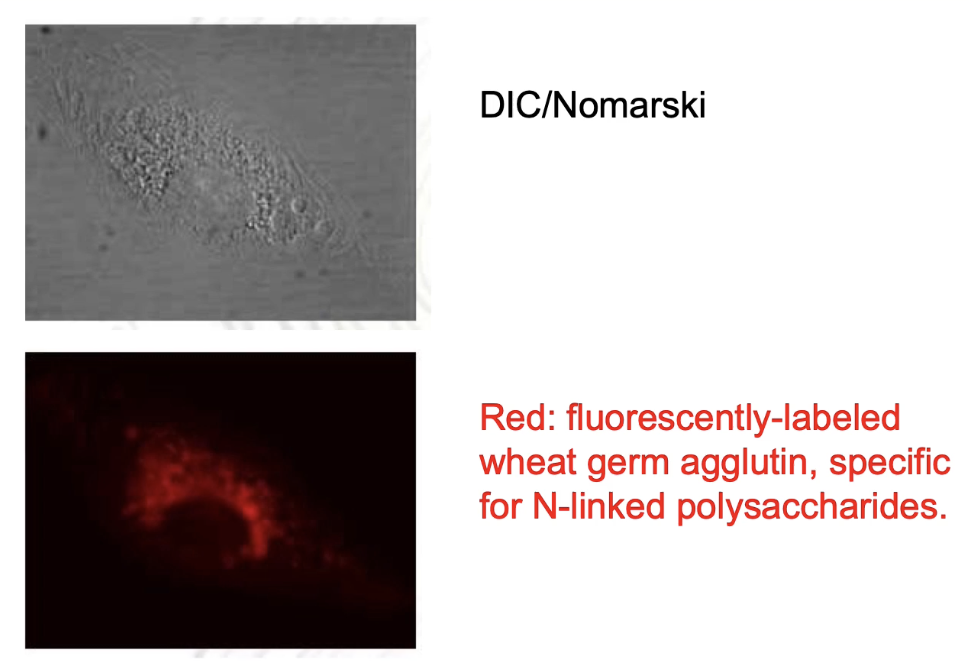
How can the Golgi complex be identified in microscopy images?
Top image: Nomarski/DIC shows all endomembranes in the cell.
To specifically label Golgi membranes: use fluorescently-labeled wheat germ agglutinin (WGA).
WGA is a lectin that binds to N-linked polysaccharides in Golgi cisternae.
Fluorescent imaging shows Golgi as a distinct subset of endomembranes.
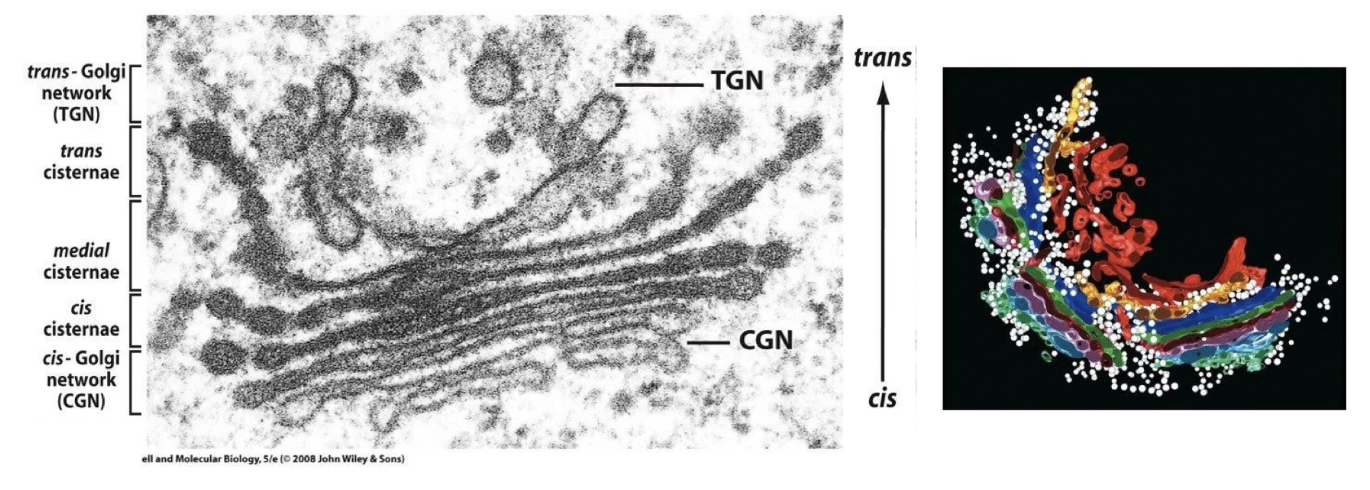
What is the structure of the Golgi complex?
Not a single organelle, but a region made of stacked, flat sacs called cisternae.
Different Golgi parts:
Cis-Golgi network: vesicles from ER forming cis-cisternae (closest to ER).
Medial cisternae: middle section.
Trans-cisternae: furthest from ER, form trans-Golgi network vesicles.
Cisternae are dynamic: vesicles constantly fuse and bud off.
Contains many mobile, spherical vesicles associated with the Golgi.
Golgi has resident proteins that modify transported proteins (post-translational modifications, PTMs).
What is anterograde transport?
Movement of proteins from the rough ER toward the cell membrane (forward direction, away from nucleus).
Vesicles help transport proteins through the Golgi complex.
Proteins pass through cis- to medial- to trans-cisternae before reaching their final destination.
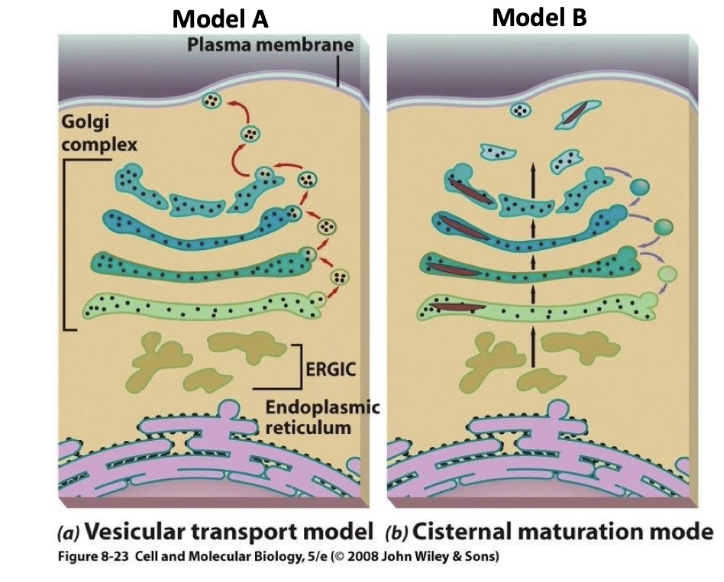
What are the two main models for protein transport through the Golgi complex?
Model A (Vesicle Transport Model):
Proteins are carried in small vesicles that move forward (anterograde) between Golgi cisternae (cis → medial → trans).
Vesicles shuttle protein cargo from one cisterna to the next.
Model B (Cisternal Maturation Model):
Proteins stay inside the cisternae, and the cisternae themselves move forward through the Golgi stack.
Vesicles move backward (retrograde) to recycle Golgi resident proteins.
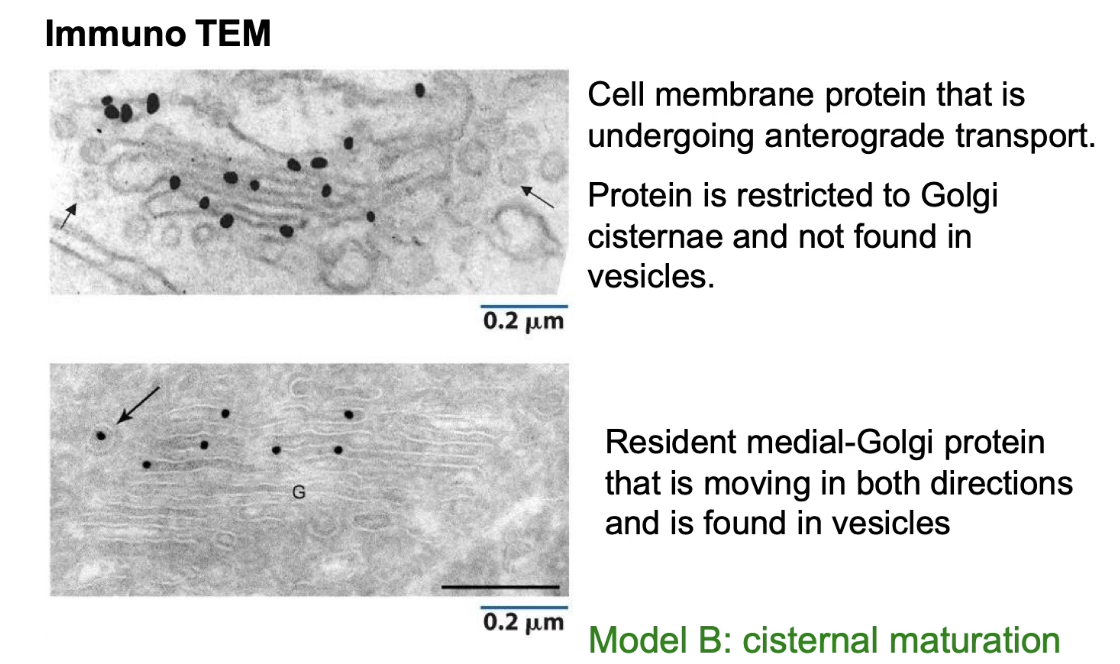
How do experiments using antibodies to specific proteins support one Golgi transport model over the other?
Using immuno-TEM, researchers label proteins and track their locations in the Golgi:
Cell membrane protein (cargo):
Found only inside cisternae, not in vesicles.
Suggests forward transport occurs inside cisternae, not via vesicles (supports Model B).
Medial-Golgi resident protein:
Found in both cisternae and vesicles.
Shows that vesicles are involved in moving resident proteins backwards to maintain Golgi structure (supports Model B).
Which model is currently accepted as the main mechanism for protein transport through the Golgi, and why?
Model B (cisternal maturation) is widely accepted because:
Forward movement of proteins inside cisternae is observed.
Vesicles primarily handle retrograde transport to recycle resident proteins.
Experimental evidence matches these patterns consistently.
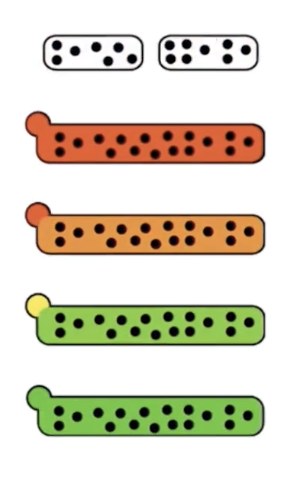
What does the cisternal maturation model explain about Golgi cisternae movement?
Golgi cisternae plus cargo move forward (anterograde) through the Golgi.
Cis-Golgi cisternae become medial-Golgi, medial-Golgi become trans-Golgi as they move.
New cis-cisternae form from vesicles coming from the ER.
The trans-Golgi network breaks down into secretory vesicles, carrying cargo proteins to their next destination.
Golgi resident proteins can get misplaced and need to be re-sorted backward by vesicles (retrograde transport).
How do vesicles move proteins during Golgi transport?
Vesicles move to, around, and away from the Golgi complex during protein transport.
Fluorescent imaging shows cargo protein moving from ER → Golgi → secretory vesicles.
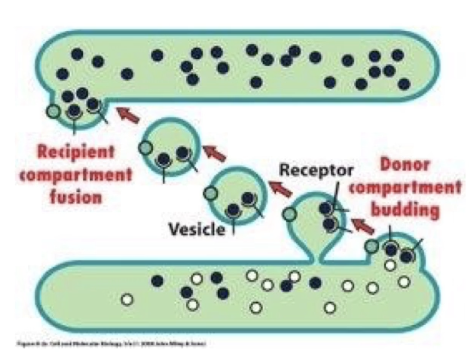
What are the four steps of vesicular trafficking?
Budding: Vesicles form by budding off from the membrane of the donor compartment.
Cargo loading: Cargo proteins are loaded into the budding vesicle via signal sequences and receptors.
Vesicle formation and release: Vesicle pinches off and is released.
Docking and fusion: Vesicle docks and fuses with the membrane of the recipient compartment.
What are the three types of vesicles involved in protein transport, and how do their coat proteins function?
Three types of coated vesicles:
Clathrin-coated vesicles
COP I-coated vesicles
COP II-coated vesicles
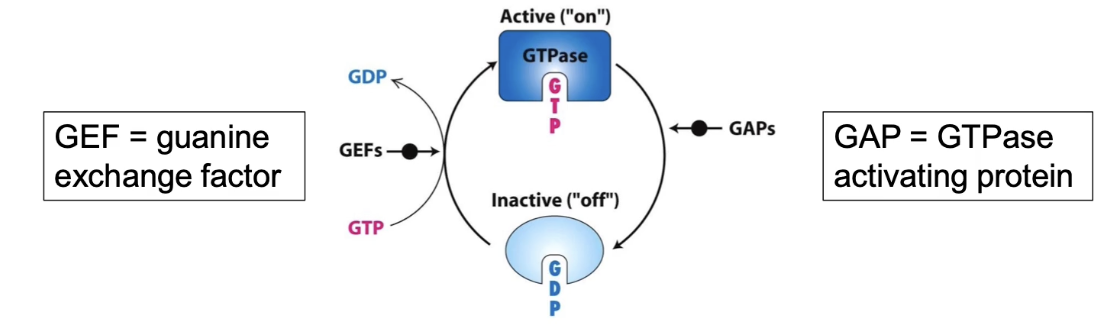
Coat proteins are small GTP-binding proteins (G-proteins) with GTPase activity.
G-proteins cycle between two states:
Active: bound to GTP
Inactive: bound to GDP
How the cycle works:
G-protein hydrolyzes GTP → GDP to become inactive (aided by GTPase-accelerating/activating proteins, GAP).
GDP is replaced by GTP to reactivate the protein (aided by guanine exchange factors, GEF).
This active/inactive cycle controls vesicle coat formation and disassembly.
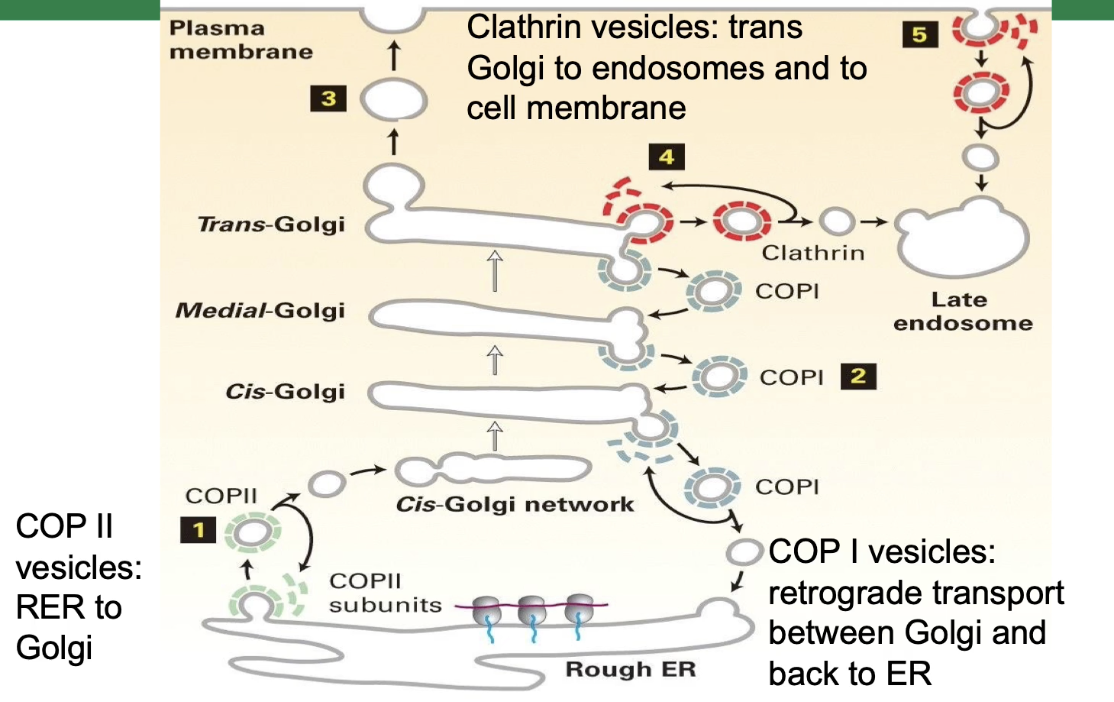
What are the three types of coated vesicles used in protein transport, and where do they function?
Clathrin-coated vesicles:
Transport from trans-Golgi network to endosomes and cell membrane
Also involved in endocytosis (bringing substances into the cell)
COP I vesicles:
Mediate retrograde transport from Golgi back to ER
COP II vesicles:
Mediate anterograde transport from rough ER to cis-Golgi network
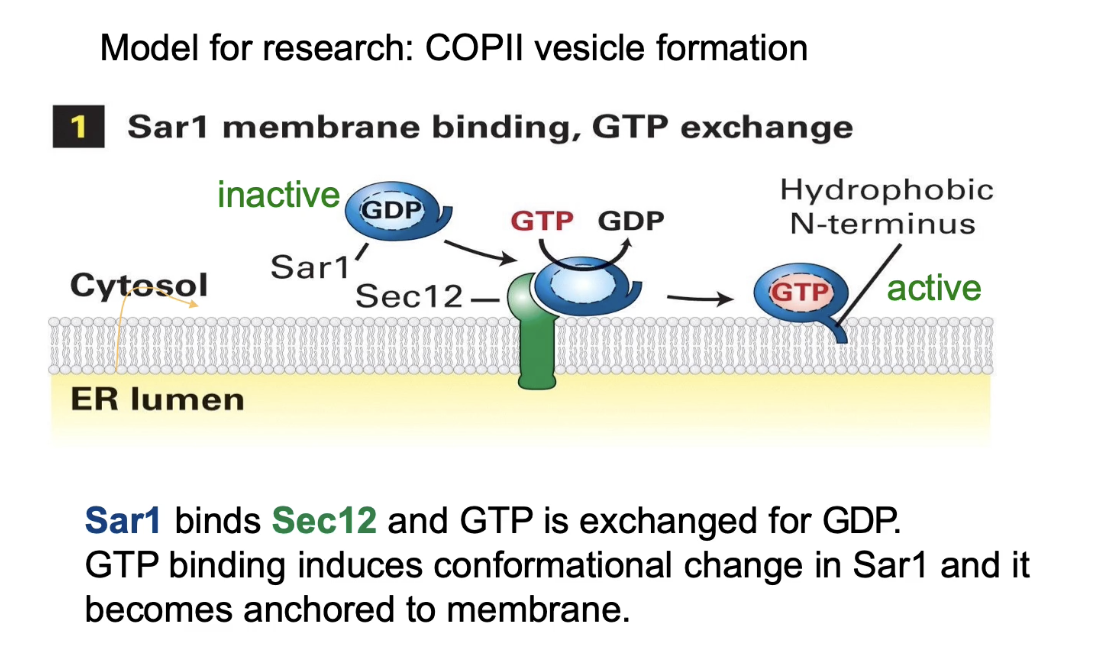
How do vesicles form during vesicular trafficking? (Using COP II vesicles as an example)
Budding starts on the donor membrane (e.g., ER)
Sar1 protein (a GTPase) is inactive in cytosol as Sar1-GDP
Sec12, a membrane protein (GEF), exchanges GDP for GTP on Sar1 → Sar1 becomes active (Sar1-GTP)
Active Sar1 changes shape, exposes a hydrophobic N-terminus, and anchors into the ER membrane
Sar1-GTP recruits COP II coat proteins from cytosol to the membrane
How do COP II coat proteins cause vesicle formation?
Sar1-GTP binds Sec23 directly and Sec24 indirectly
Sec13 and Sec31 coat proteins accumulate next
The COP II coat proteins have a natural curve that bends the membrane
This bending causes the membrane to form a bud, leading to vesicle formation
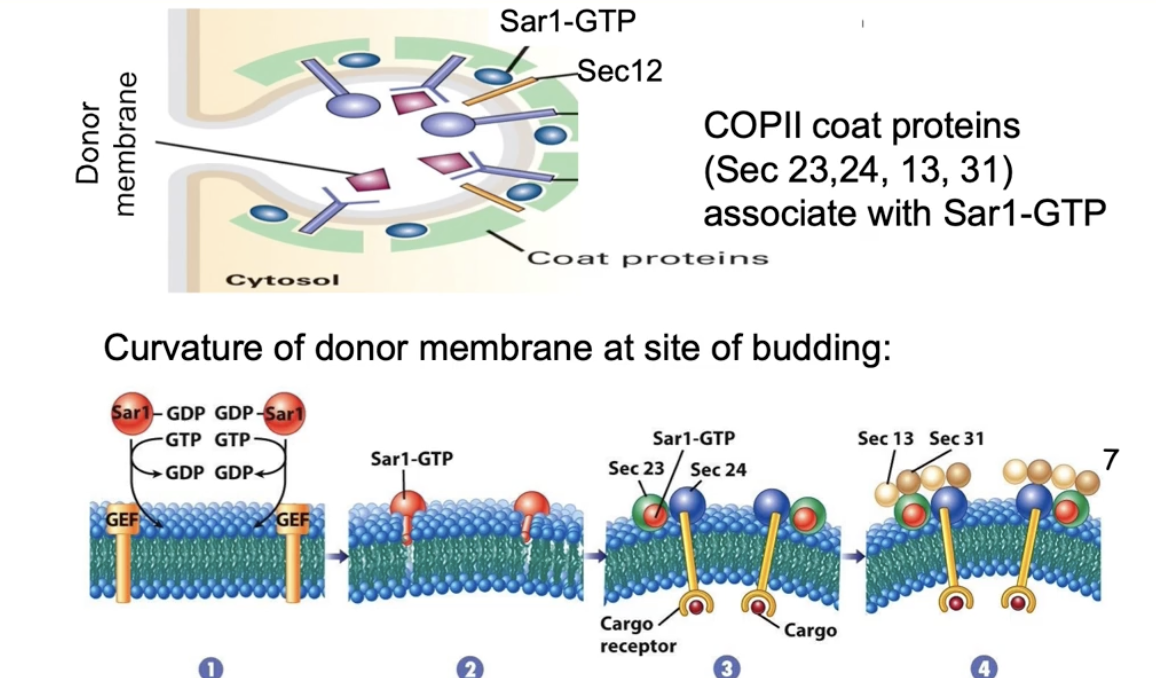
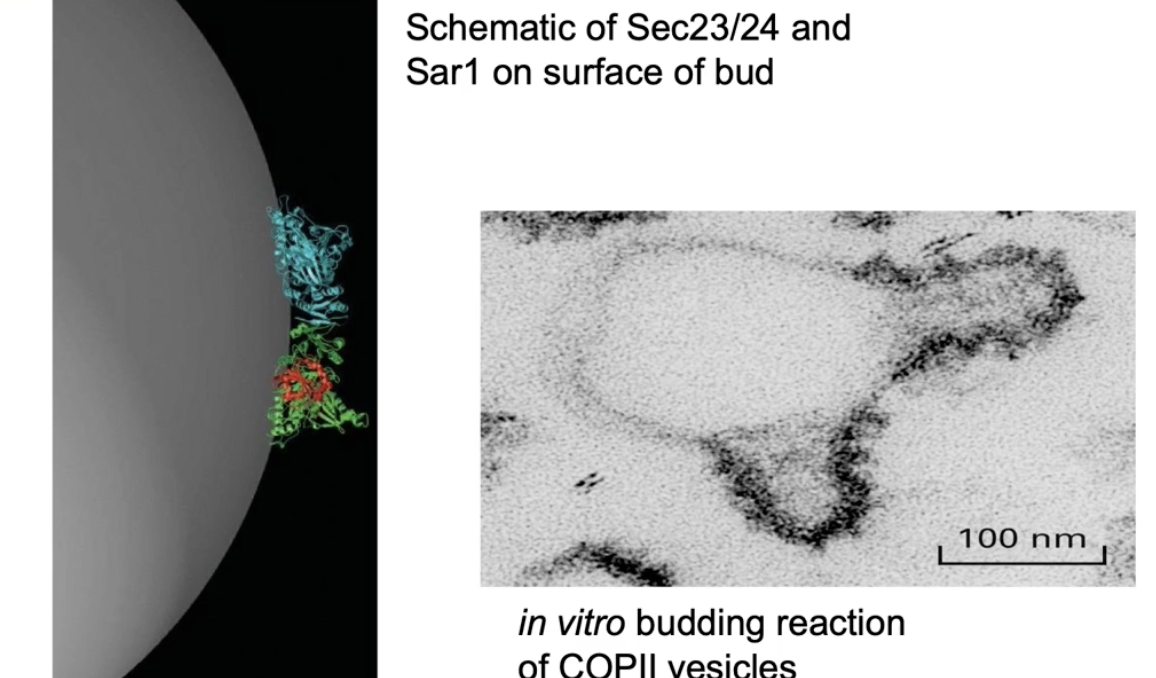
How do Sec23, Sec24, and Sar1 contribute to vesicle formation?
Sec23 and Sec24 form a curved dimer
Sar1-GTP anchors into the ER membrane
Binding of Sec23/24 to membrane-bound Sar1 forces membrane curvature
Curved membrane leads to vesicle budding
Seen in lab using ER membrane micelles + COPII proteins + Sar1-GTP
Budding only happens where COPII coat proteins are present (confirmed via immuno-TEM)
How is vesicle budding from the Golgi (COP I or clathrin) different from COP II vesicles?
Uses ARF G-protein instead of Sar1
ARF functions similarly to Sar1
ARF is required for forming COP I and clathrin-coated vesicles
Vesicles still bud via membrane curvature and coat protein assembly
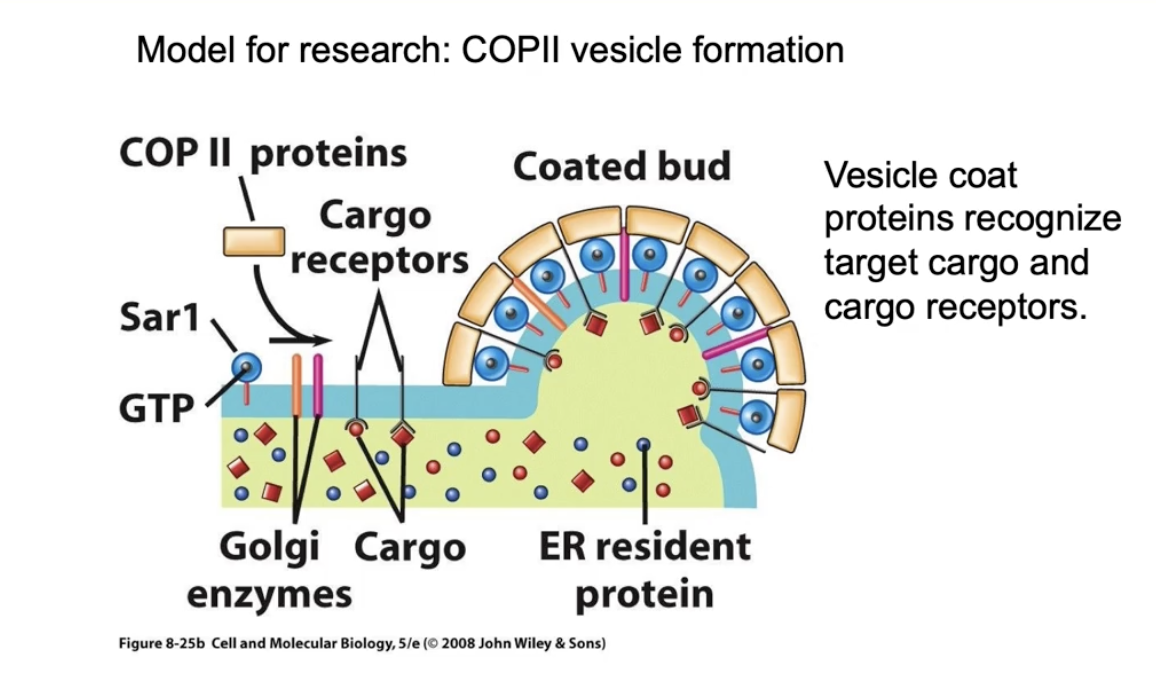
What happens during Step 2 of vesicle formation – cargo loading?
Cargo accumulates inside the curved bud on the ER membrane
Cargo receptors bind soluble proteins inside the bud
Transmembrane cargo (e.g., Golgi enzymes) also included
Coat proteins bind to cytosolic domains of cargo receptors → helps gather specific cargo
Some ER resident proteins may get in by accident, but not concentrated
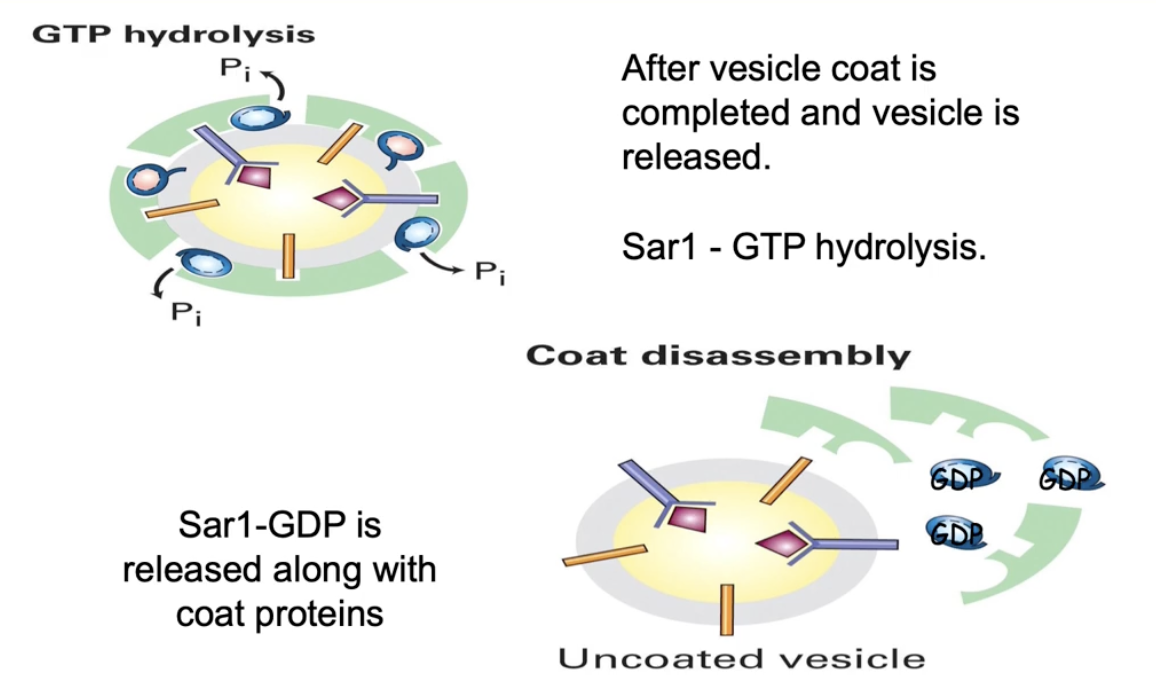
What happens after cargo is loaded into a vesicle during vesicle formation?
GTP hydrolysis converts Sar1-GTP → Sar1-GDP
Sar1-GDP detaches from membrane, triggering coat protein release
This process is called uncoating
Results in a naked (uncoated) vesicle loaded with cargo
Motor proteins recognize the uncoated vesicle
Vesicle is transported along microtubules to the recipient membrane
How can we study short-lived vesicle formation steps like the coated vesicle stage?
Normally, coated vesicles are hard to capture because the stage is very brief
To freeze this step, prevent uncoating by:
Using a non-hydrolyzable GTP (keeps Sar1 in active GTP-bound form)
Using a Sar1 mutant with impaired GTPase activity
Result: Sar1-GTP remains bound, so the coat stays on the vesicle
This prevents vesicle transport, docking at target membrane and cargo unloading since coat proteins block necessary surface proteins needed for these steps
Allows coated vesicles to accumulate and be visualized under TEM (black dots = coat proteins)
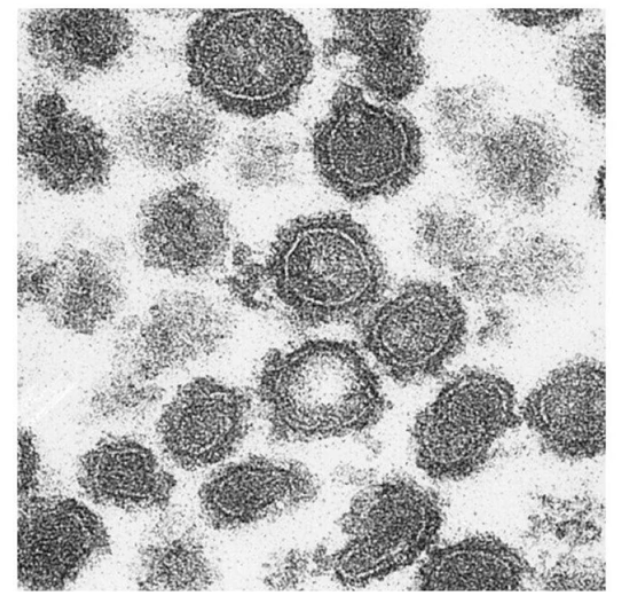
What proteins make up the clathrin coat on clathrin-coated vesicles?
Made of polyhedral lattice using:
Clathrin heavy chains (pink)
Clathrin light chains (blue)
Adaptor proteins
3 heavy + 3 light chains = a tri-scallion
Tri-scallions self-assemble into a lattice on the budding membrane
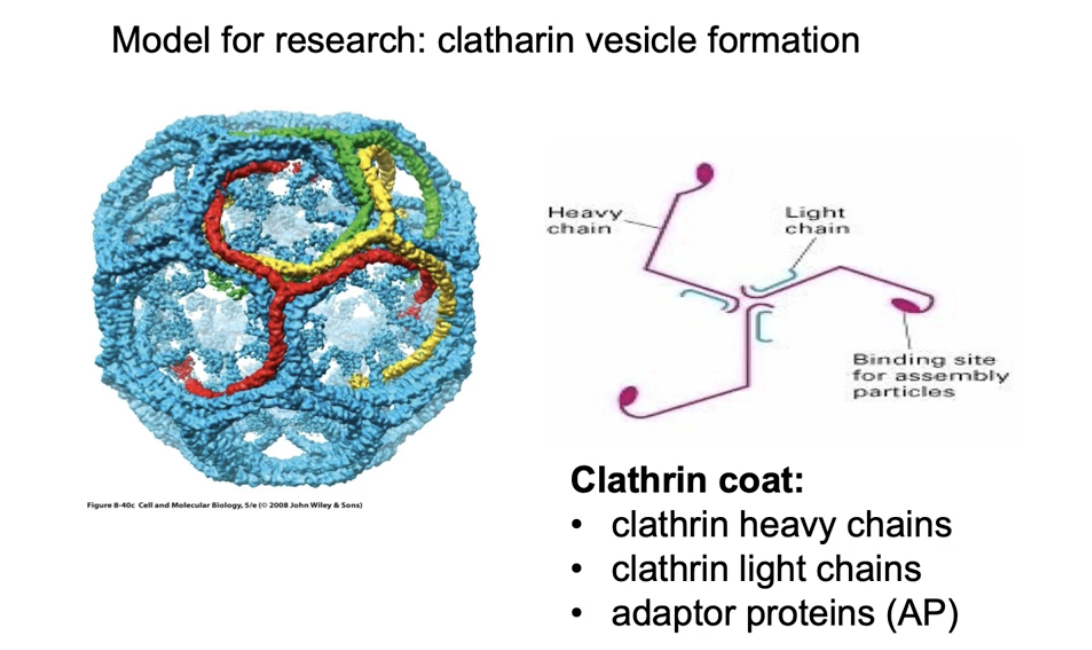
What is the role of dynamin in vesicle release?
Dynamin is a G-protein involved in clathrin-coated vesicle release
In its active form (bound to GTP), it:
Binds the neck of the budding vesicle
Hydrolyzes GTP → GDP
Changes shape, tightening the neck and causing vesicle release
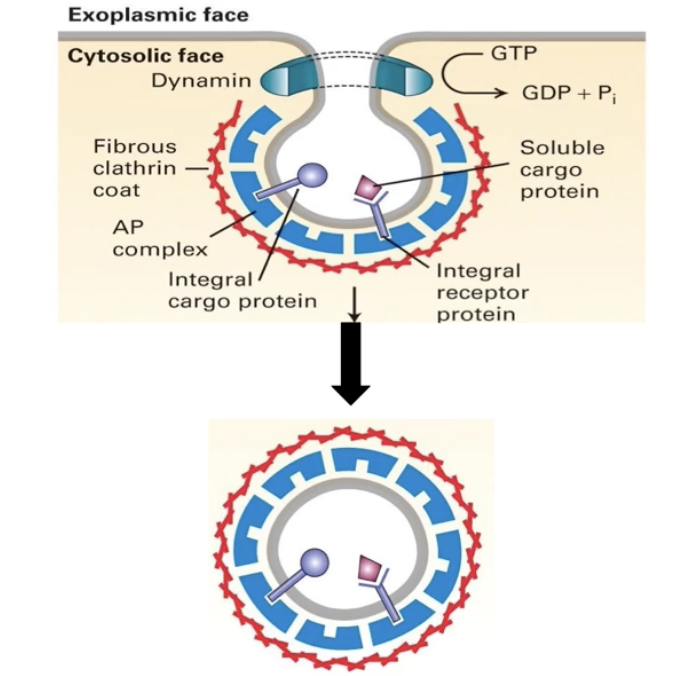
How can we visualize dynamin’s role in vesicle budding using experimental tools?
Use non-hydrolyzable GTP or dynamin mutants (block GTP hydrolysis)
This causes:
Dynamin to accumulate around vesicle neck creating long neck
Vesicle to stay attached (no release)
Seen under TEM:
Black dots = antibodies to dynamin
Dynamin wraps around the neck in ring-like structures
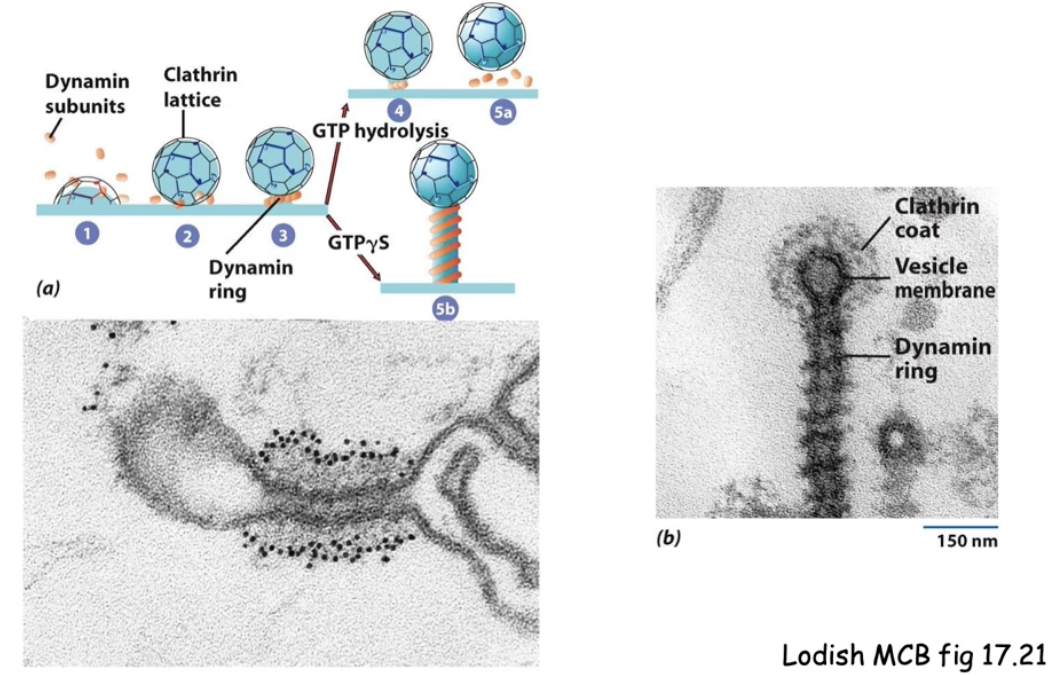
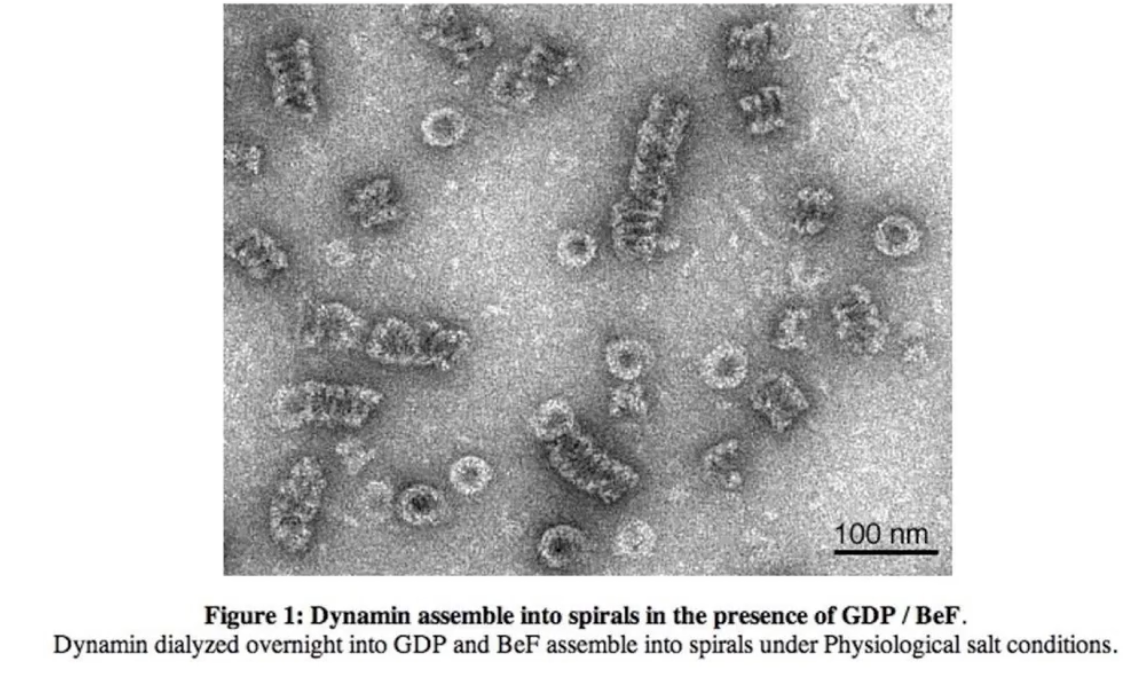
What does electron microscopy (EM) reveal about dynamin structure in the presence of GDP?
Dynamin forms spiral (corkscrew-like) polymers
These helical structures wrap around the vesicle neck
Seen clearly when GDP is present (post-GTP hydrolysis)
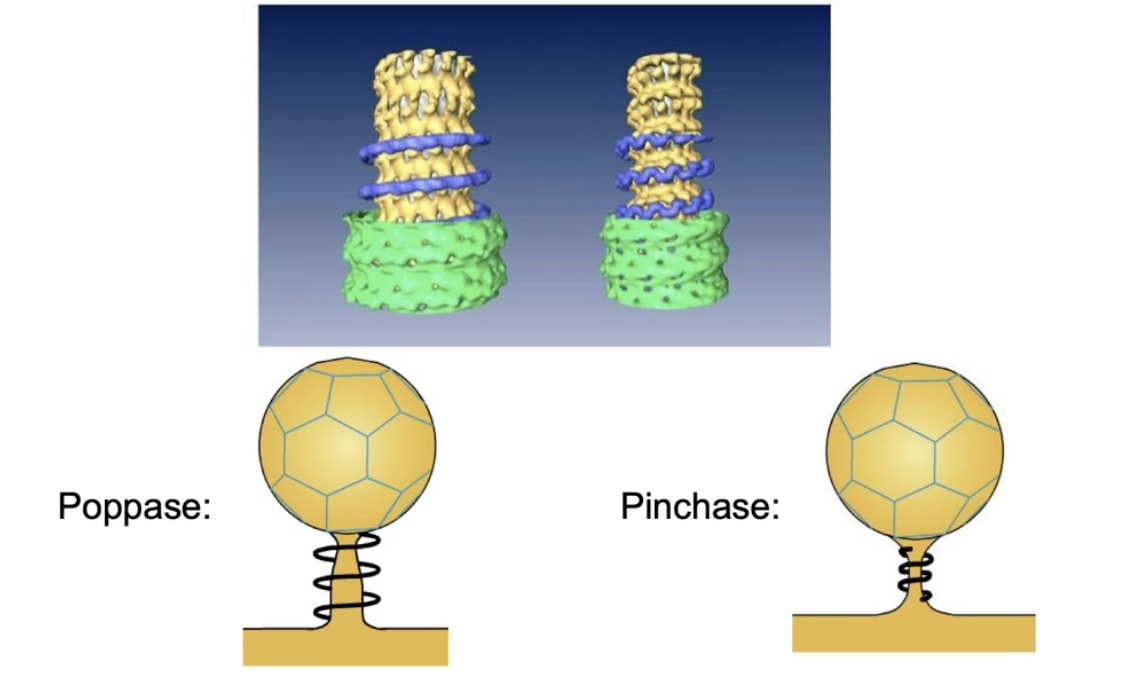
What are the two models for how dynamin causes vesicle release?
Poppase model:
Dynamin helices elongate
This pushes the vesicle away from the membrane
Pinchase model:
Dynamin helices constrict (tighten)
This squeezes the neck of the vesicle, causing release
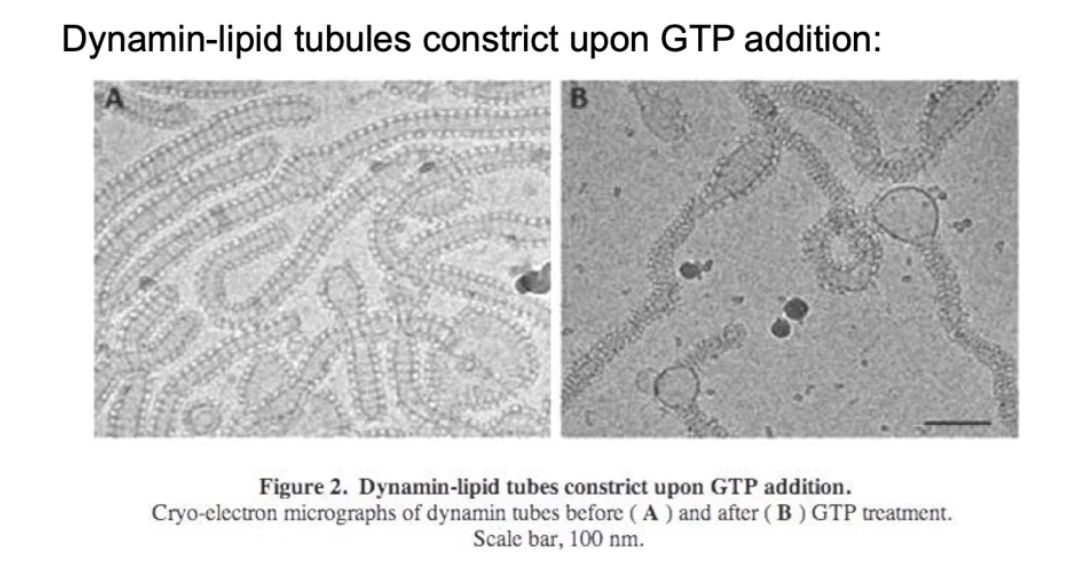
What experimental evidence supports the pinchase model?
In vitro experiments use lipid tubes as vesicle neck models
Adding GTP causes dynamin to tighten around the lipid tube
This narrows the internal diameter of both the dynamin spiral and lipid tube
Suggests that dynamin constricts the membrane = pinchase support
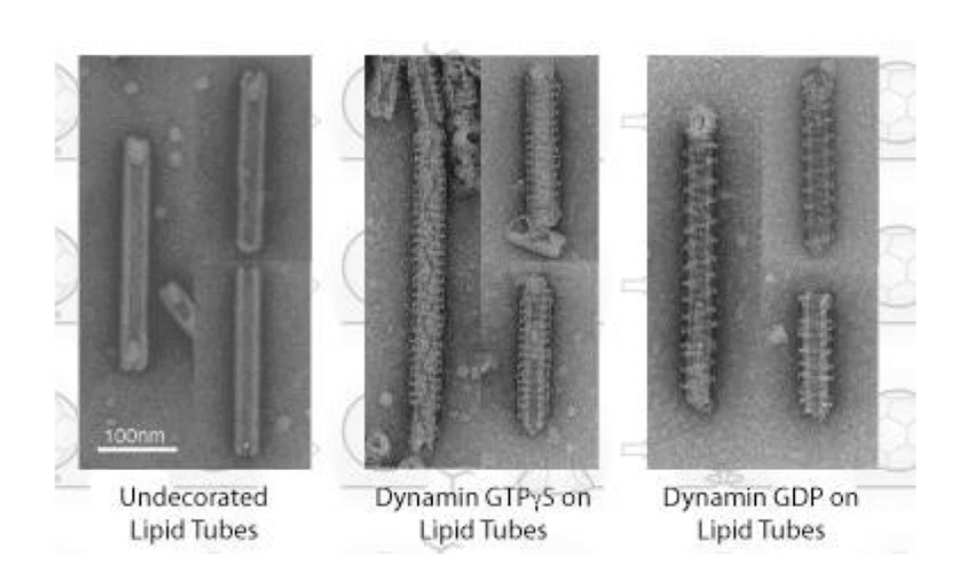
What experimental evidence supports the poppase model?
EM images show lipid tubes with:
No dynamin (left)
Dynamin + non-hydrolyzable GTP (middle)
Dynamin + GDP after GTP hydrolysis (right)
After GTP hydrolysis:
Helical rings are more spaced apart
Indicates elongation of the polymer
This supports the poppase model
Which model (pinchase or poppase) is correct for dynamin function?
Current research suggests:
Both models may be correct
Dynamin likely uses a combination of constriction (pinchase) and elongation (poppase)
This dual mechanism helps ensure efficient vesicle release
How is dynamin studied in fruit flies, and what is its role in neurons?
Studied using Drosophila melanogaster (fruit fly)
Focus: Vesicles formed during endocytosis in presynaptic neurons
Dynamin helps form vesicles at the cell membrane
These vesicles carry neurotransmitters from cytosol
Vesicles fuse with membrane to release neurotransmitters via exocytosis
Neurotransmitters bind to receptors on post-synaptic cells to send signals
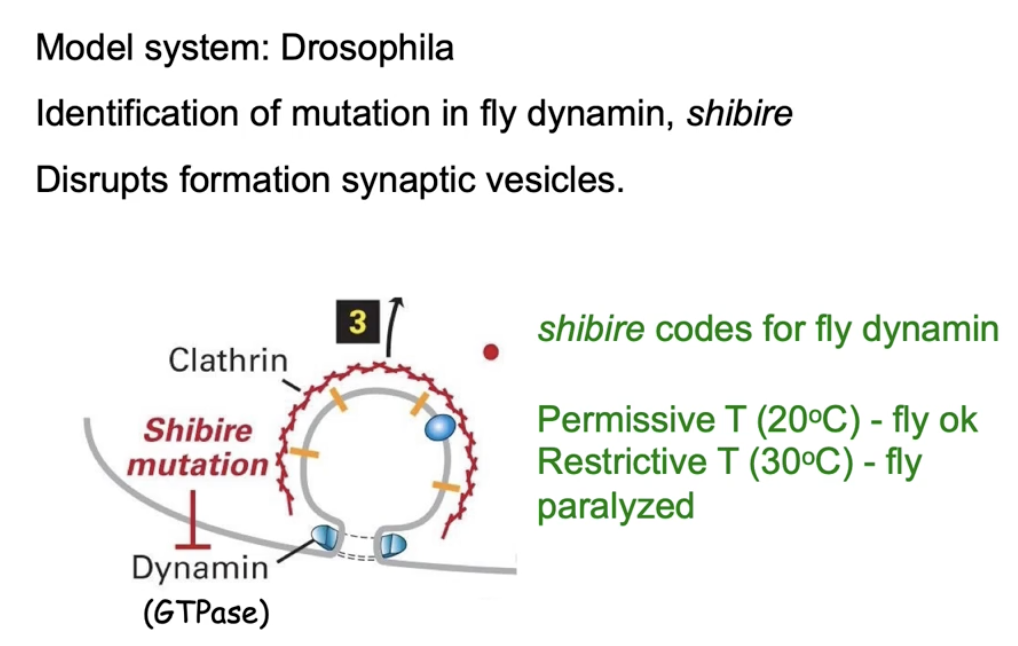
The shibire gene in flies makes the dynamin protein
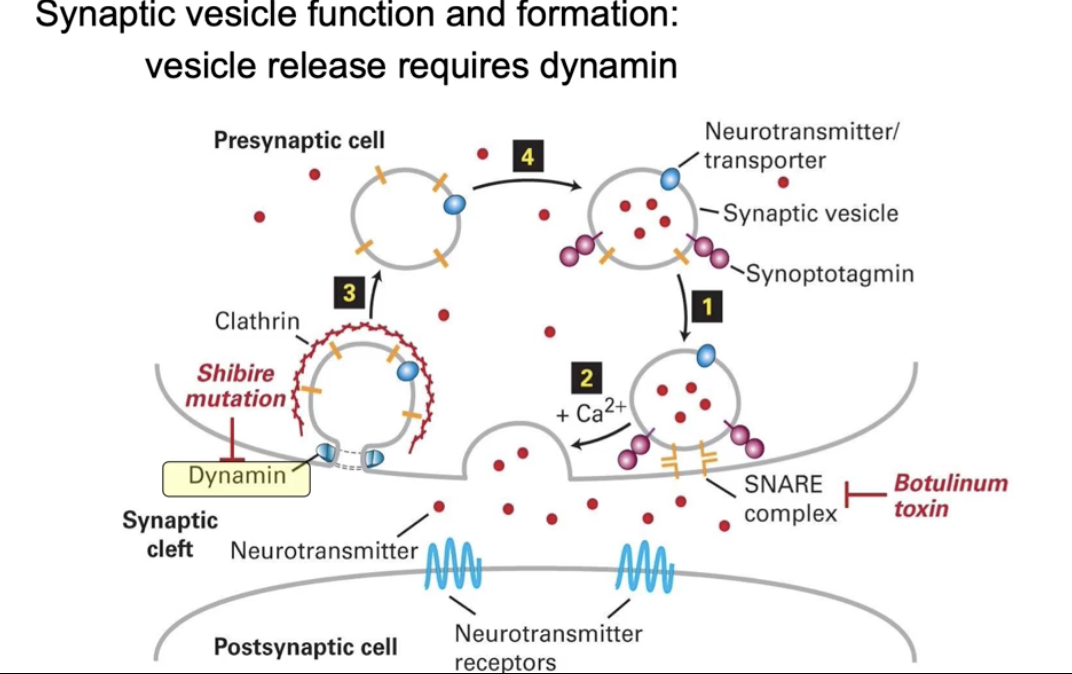
What happens to fruit flies with a temperature-sensitive mutation in the shibire gene at different temperatures?
Permissive temp (25°C):
Dynamin folds properly
Vesicle formation occurs
Neurotransmitters released
Flies behave normally
Restrictive temp (30°C):
Dynamin becomes misfolded (non-functional)
No vesicle formation
No neurotransmitter release
Flies become paralyzed
What happens to shibire mutant fruit flies as temperature increases and decreases?
At normal temp (25–27°C):
Dynamin works → vesicles form → flies move normally
As temp increases (29°C):
Dynamin begins to unfold
Vesicle formation slows → flies start to fall
At high temp (33°C):
Dynamin non-functional
No vesicle formation → no neurotransmitter release → paralysis
When temp is lowered again:
Dynamin refolds and works again
Vesicle transport resumes → flies recover from paralysis
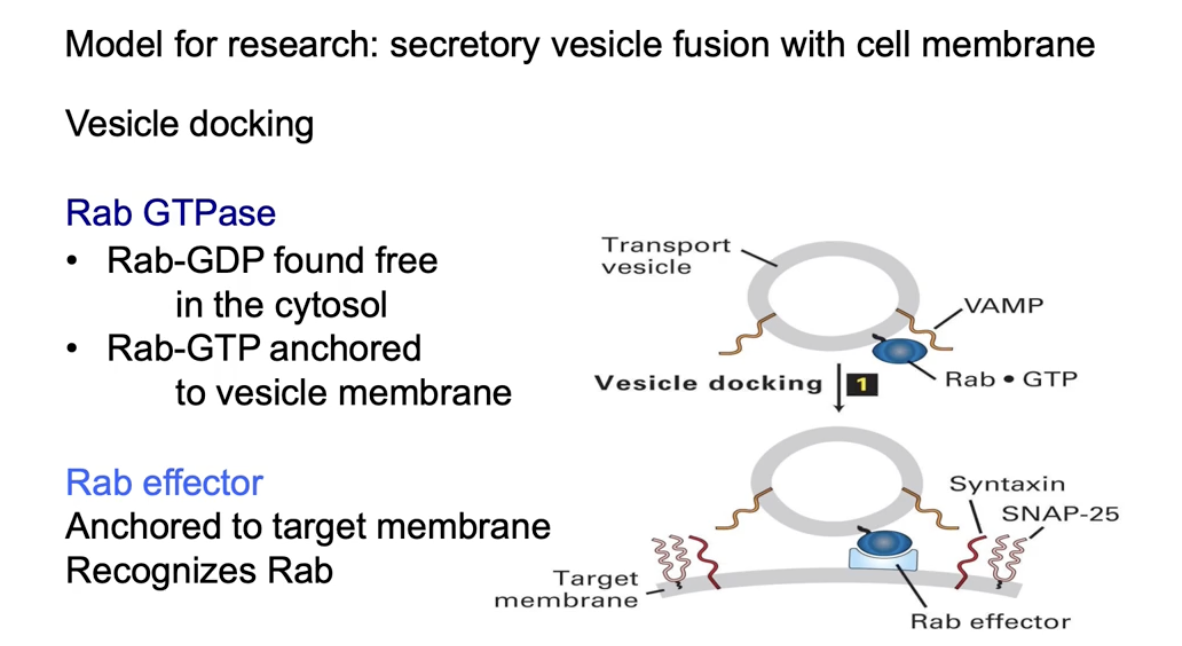
What role does Rab GTPase play in vesicle docking and cargo release?
Rab-GDP:
Inactive form
Free in the cytosol
Rab-GTP:
Active form
Bound to vesicles by hydrophobic anchor
Function of Rab-GTP:
Binds to Rab effector on the target membrane
Helps dock the vesicle at the correct location
Docking vs. Fusion:
Docking aligns vesicle with target
Fusion is still needed to release the cargo inside
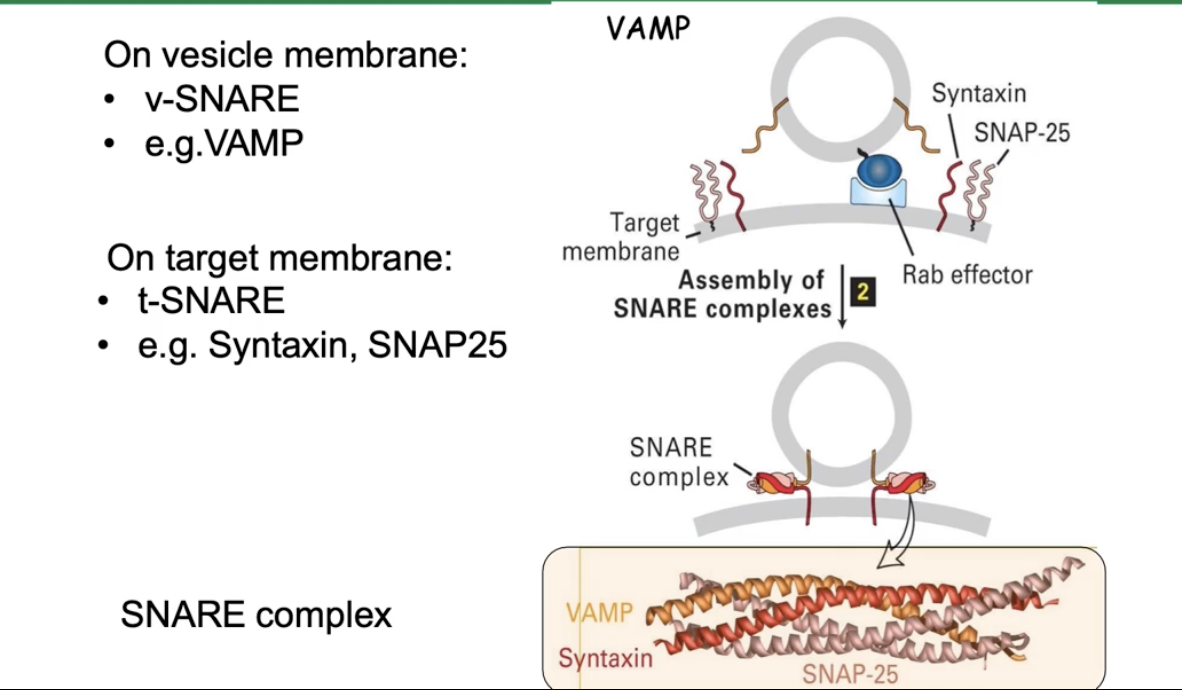
How do SNARE proteins mediate vesicle fusion?
v-SNAREs (e.g., VAMP) on vesicle membrane
t-SNAREs (e.g., syntaxin, SNAP25) on target membrane
SNAREs form a four-helix bundle:
2 helices from SNAP25
1 helix from syntaxin
1 helix from VAMP
The bundle pulls membranes close
This close contact causes membrane fusion
Fusion releases vesicle cargo into the target compartment
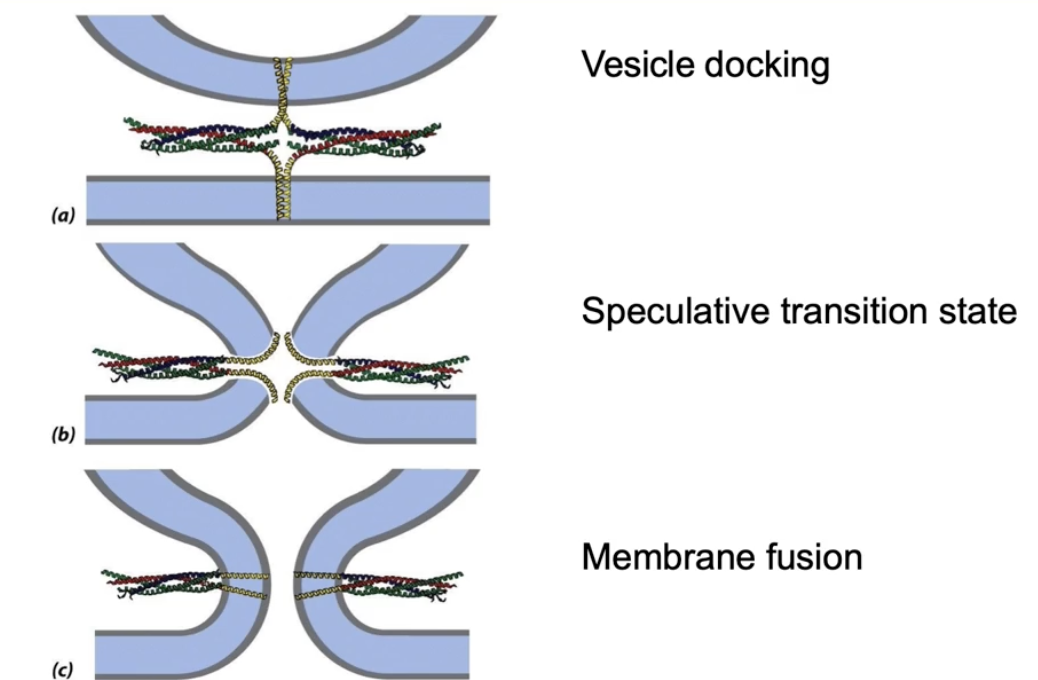
How do SNARE proteins mediate membrane fusion?
SNARE helices spiral tightly, pulling vesicle and target membranes close
Transmembrane domains pulled apart as cytosolic domains spiral
Membranes fuse by forming a hole where contents mix
Membranes reseal to become continuous, allowing cargo release
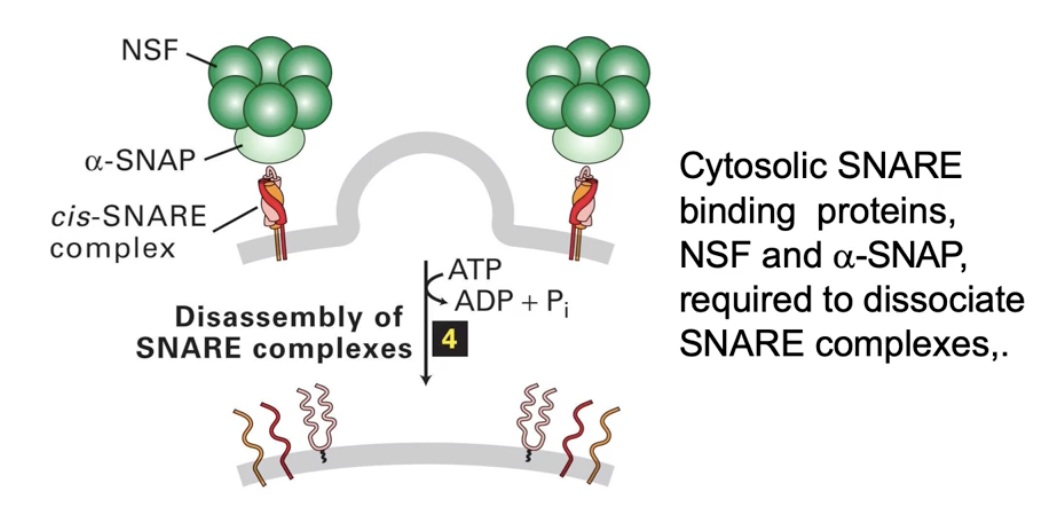
What happens to SNARE proteins after membrane fusion?
SNARE complex must be disassembled
NSF and alpha-SNAP proteins bind to SNARE complex
They unwind the 4-helix SNARE bundle
Freed SNAREs recycle and diffuse in membranes for reuse
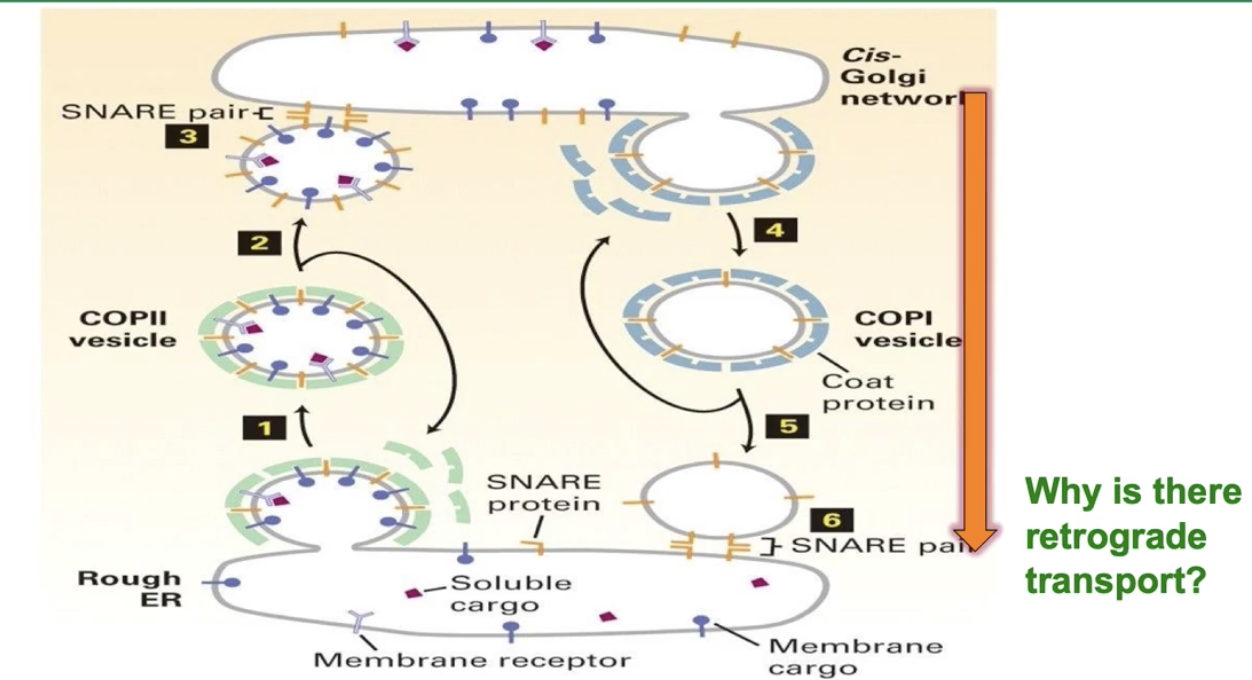
Why does the cell need retrograde transport?
To return ER resident proteins accidentally sent to Golgi
To recycle SNAREs and COPII cargo receptors for reuse
To send unfolded/misfolded proteins back to ER for refolding or degradation
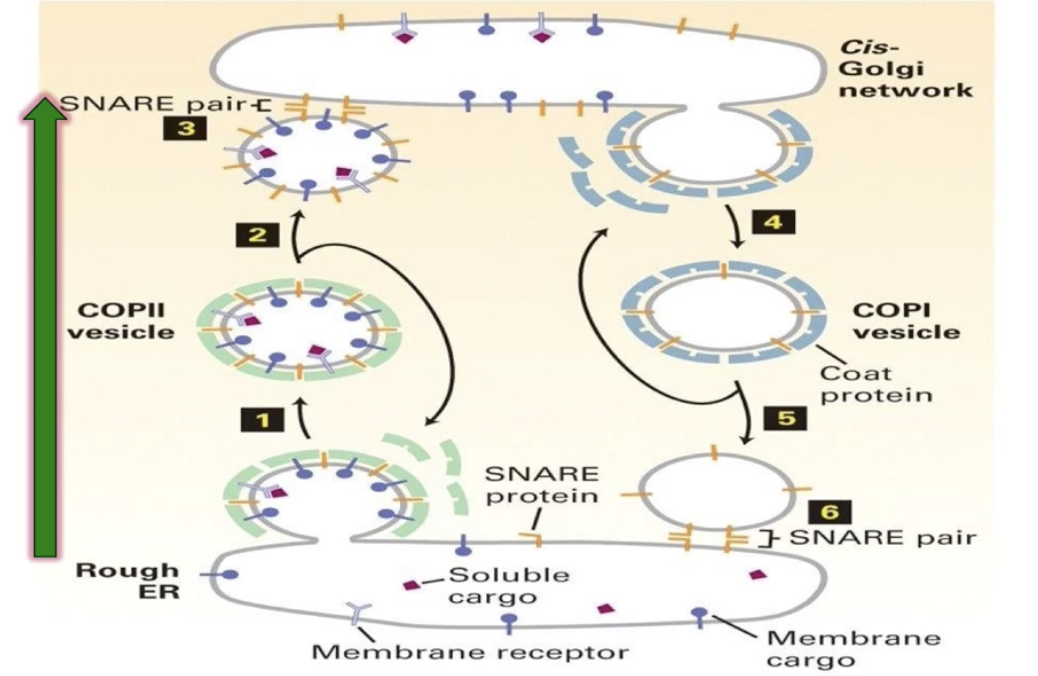
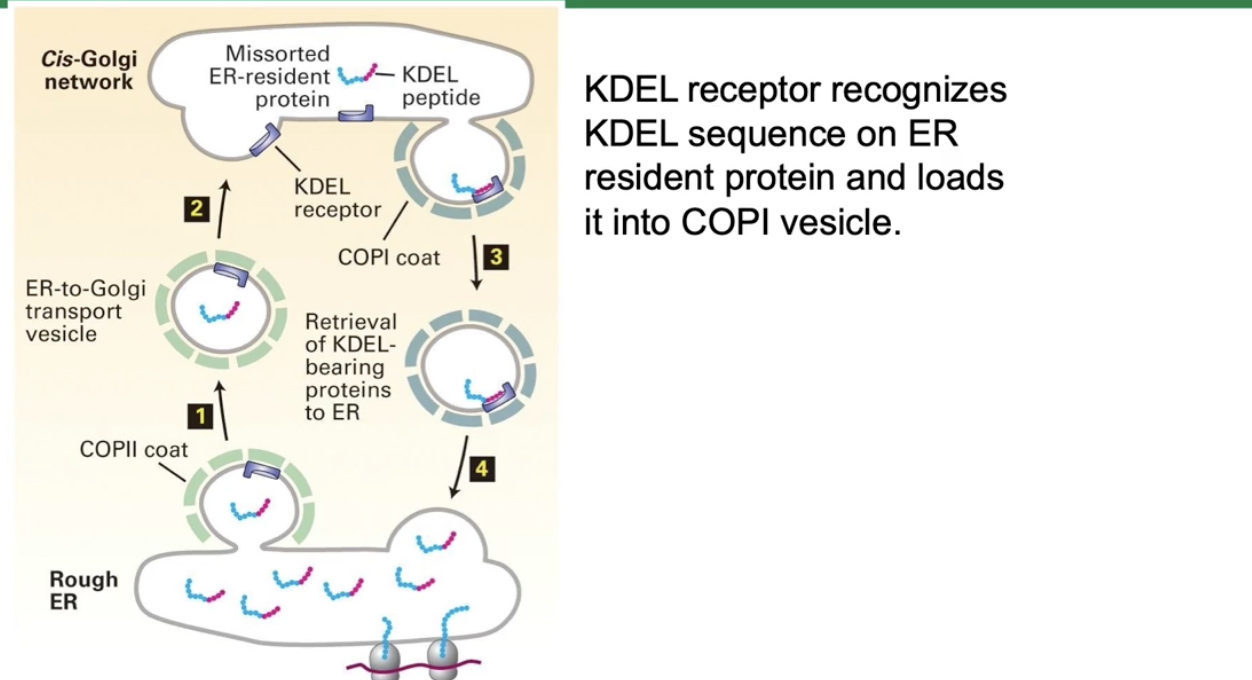
How are ER resident proteins recognized and returned to the ER via COPI vesicles?
ER resident proteins have specific signal sequences like:
KDEL (Lys-Asp-Glu-Leu) for soluble ER resident proteins
Lysine-rich sequences for ER membrane proteins
Aspartate-X-glutamate on COPII cargo receptors
The KDEL receptor in the Golgi binds proteins with the KDEL signal
The receptor-protein complex is loaded into COPI vesicles
COPI vesicles transport these proteins back to the ER for proper localization
What happens if there is a mutation in clathrin coat proteins?
Proteins accumulate in the trans-Golgi (class E secretory mutant phenotype)
Vesicle transport and cargo sorting is disrupted