SOLUBILITY EQUILIBRIA | 4.3
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Solution
homogenous mixture of 2 or more substances
Solute
substance(s) present in
the smaller amount(s)
Solvent
substance present in the
larger amount
Solution Process
molecular view of the formation of solution
Stirring (Agitation)
Surface Area
Temperature
Factors Affecting Rate of Dissolution
Solubility
Maximum amount of solute that can be dissolved in a given
amount of solvent at a specified temperature
Molar solubility (s)
- the number of moles of
solute dissolved in 1 L of
a saturated solution.
- moles solute/L of solution
- Unit: M
saturated solution
Dissolved solute = solubility
unsaturated solution
Dissolved solute < solubility
supersaturated solution
Dissolved solute > solubility
Nature of Solute and
Solvent
Pressure
Temperature
Factors Affecting Solubility
↑temperature, ↓ solubility
relationship of temperature and solubility and gas phase
Saturated Solutions
any undissolved solids are in
equilibrium with the saturated
solution (liquid)
undissolved solute and saturated solution
equilibrium exists between an
**** and its *****
Unsaturated solution No precipitate
Q < Ksp
Saturated solution
Q = Ksp
Saturated solution, Supersaturated solution, Precipitate will form
Q > Ksp
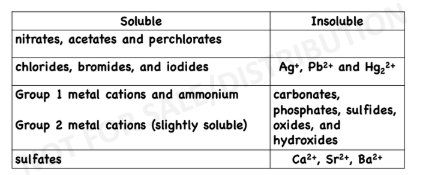
rules to remember in dissolution equations
COMPLEX IONS
ions that contains a
central metal cation
bonded to one or more
molecules or ions
- usually participated in
by transition metal
cations (have more
than one oxidation
state)