Factors that Affect Reaction Rate: Temperature, Catalysts, Concentration of Reactants
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Catalyst
substance that speeds up the rate of a chemical reaction
Are Catalysts consumed in a reaction?
Catalysts are not consumed
Is the equilibrium of a chemical reaction affected in any way by catalysts
Catalysts do not affect the equilibrium of a chemical reaction
Reaction Temperature
reaction rates generally increase as temperature is increased
The atoms of a substance have energy. T/F
True
Some atoms have a large amount of energy T/F
True
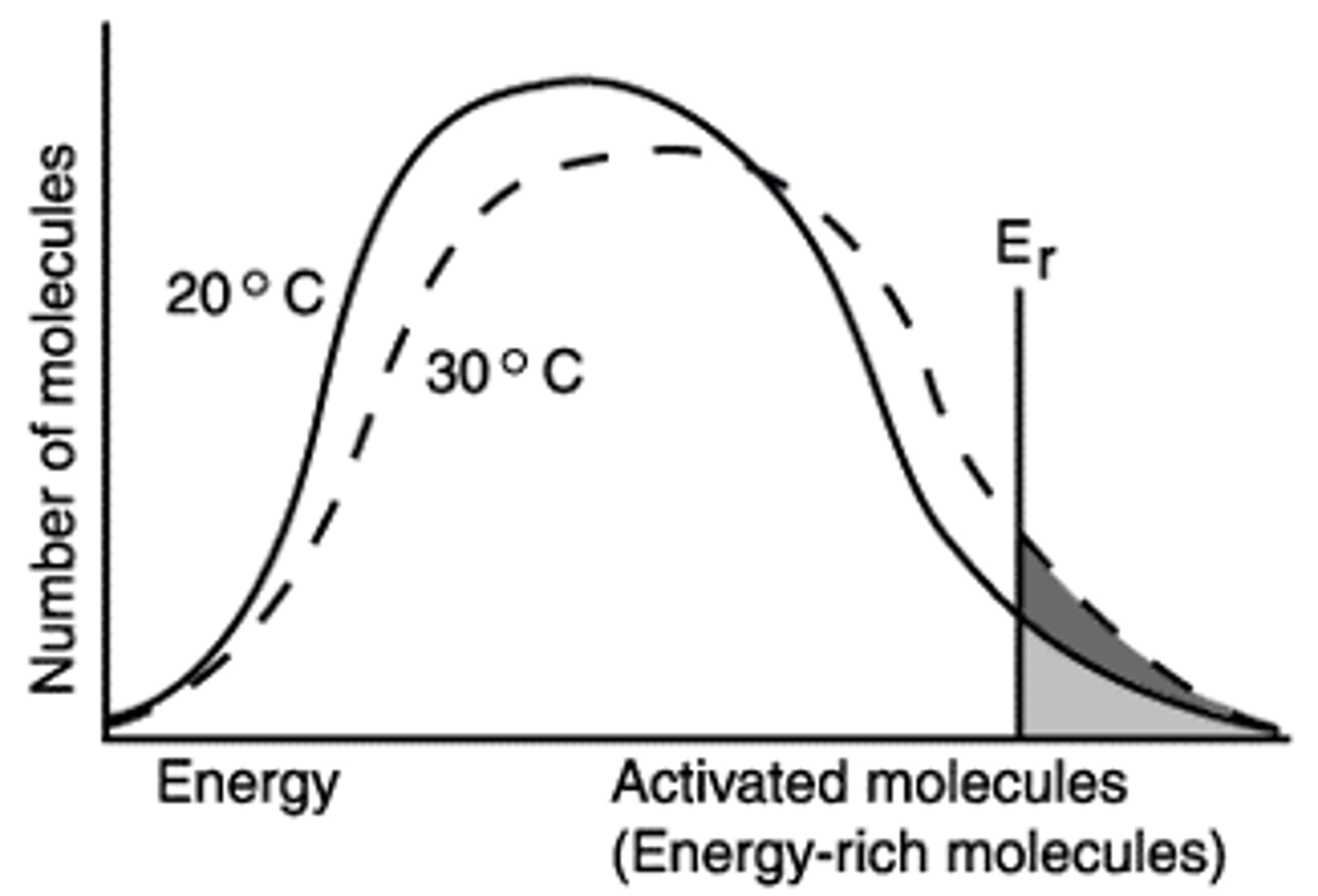
>Er
This means molecules have the energy required to react.
The rate of endothermic reactions ____________________ as temperature is raised
increases
What is the significance of Er in the diagram
For exothermic reactions what happens to the energy level of the products vs the energy level of the reactants.
the energy level of the products is lower than the energy level of the reactants.
For endothermic reactions what happens to the energy level of the products vs the energy level of the reactants.
the energy level of the products is higher than the energy level of the reactants.
Does reactant concentration increase reaction rate? T/F
True
T/F raising the pressure on reactants in the form of a gas will increase reaction rate
True
The effect of a ten degree increase in temperature is shown in the increased number of molecules having the energy required to react (>Er).
The figure shows that when the temperature is increased ten degrees, the number of molecules with the minimum energy to react, Er, has increased by the amount represented by the dark region of the graph.
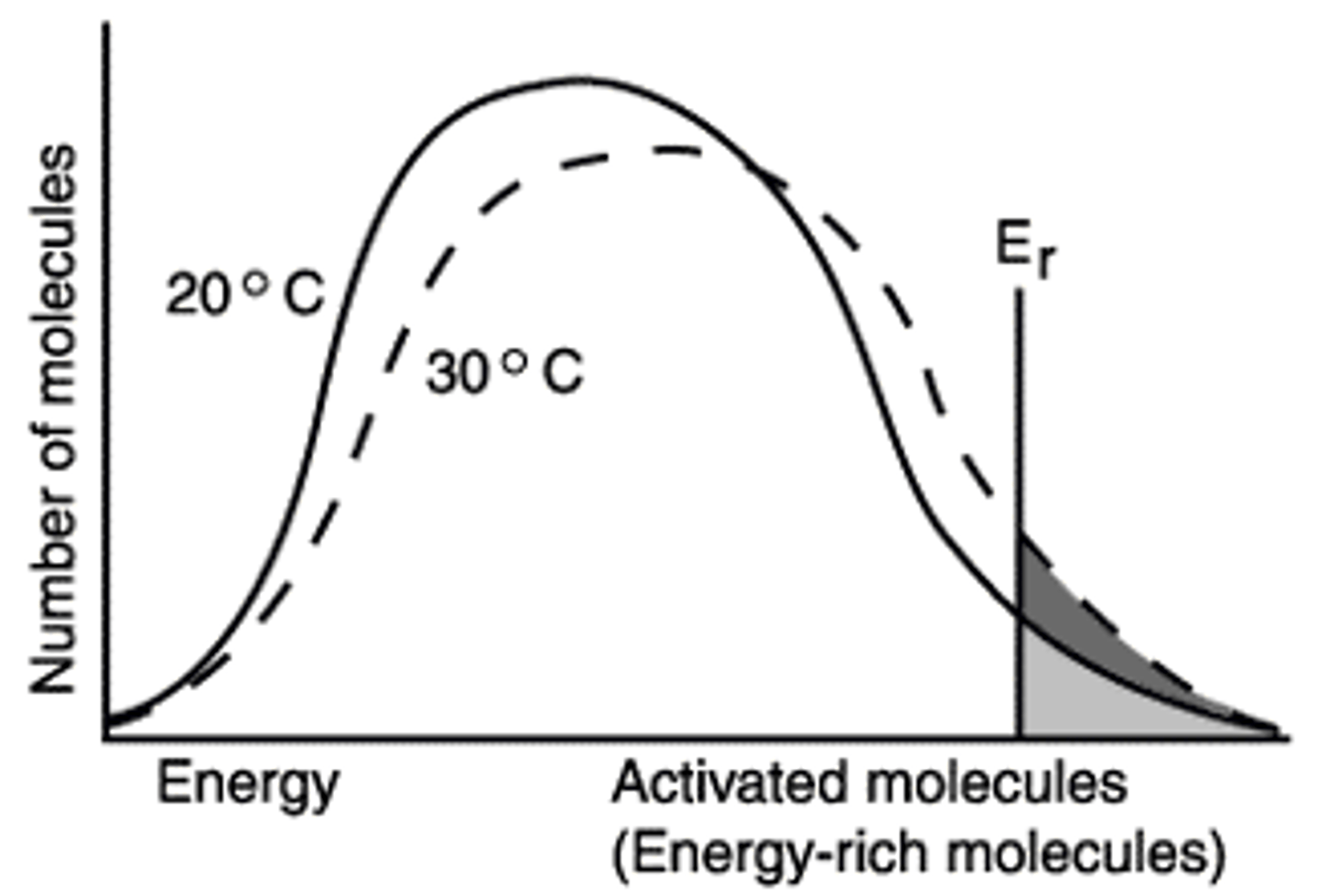
What is the significance of Er in the diagram?