Lecture 4: Enzyme Regulation Part 1
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
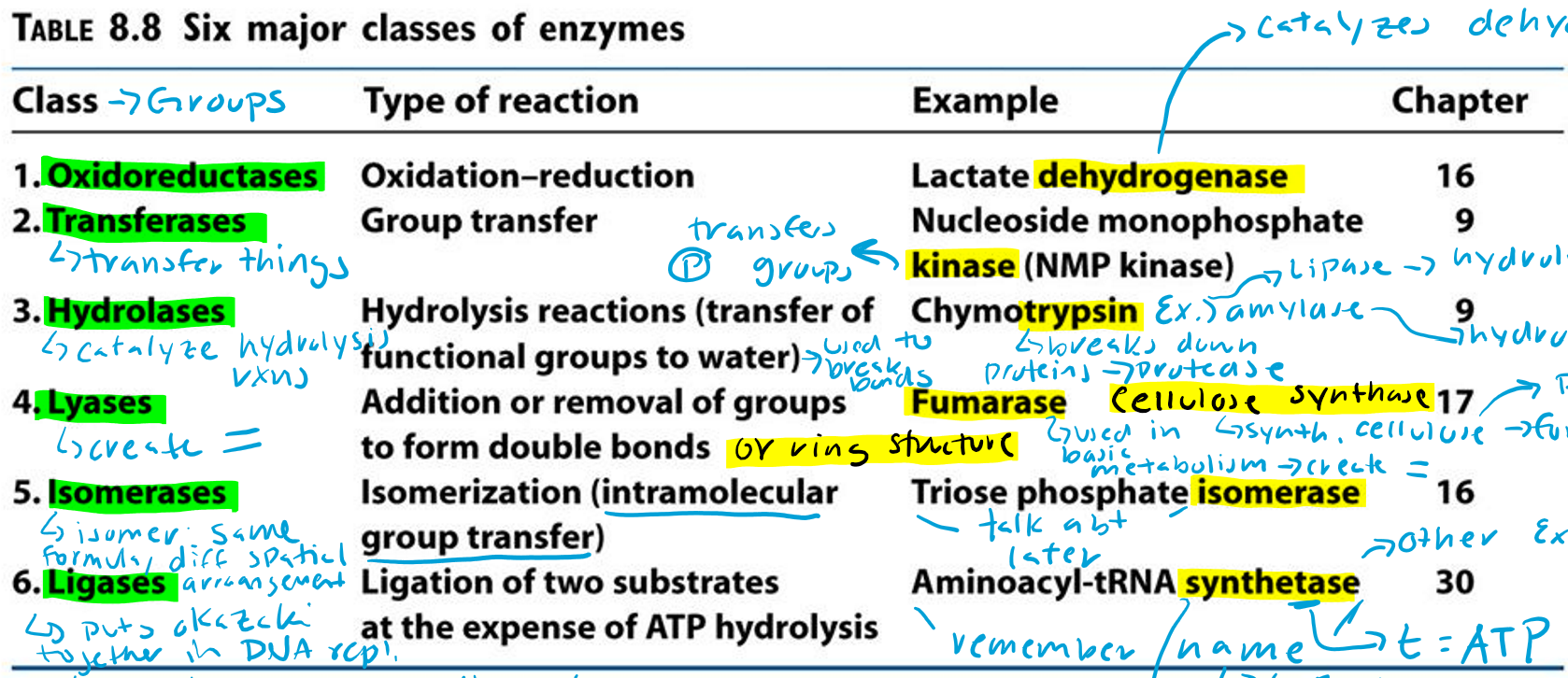
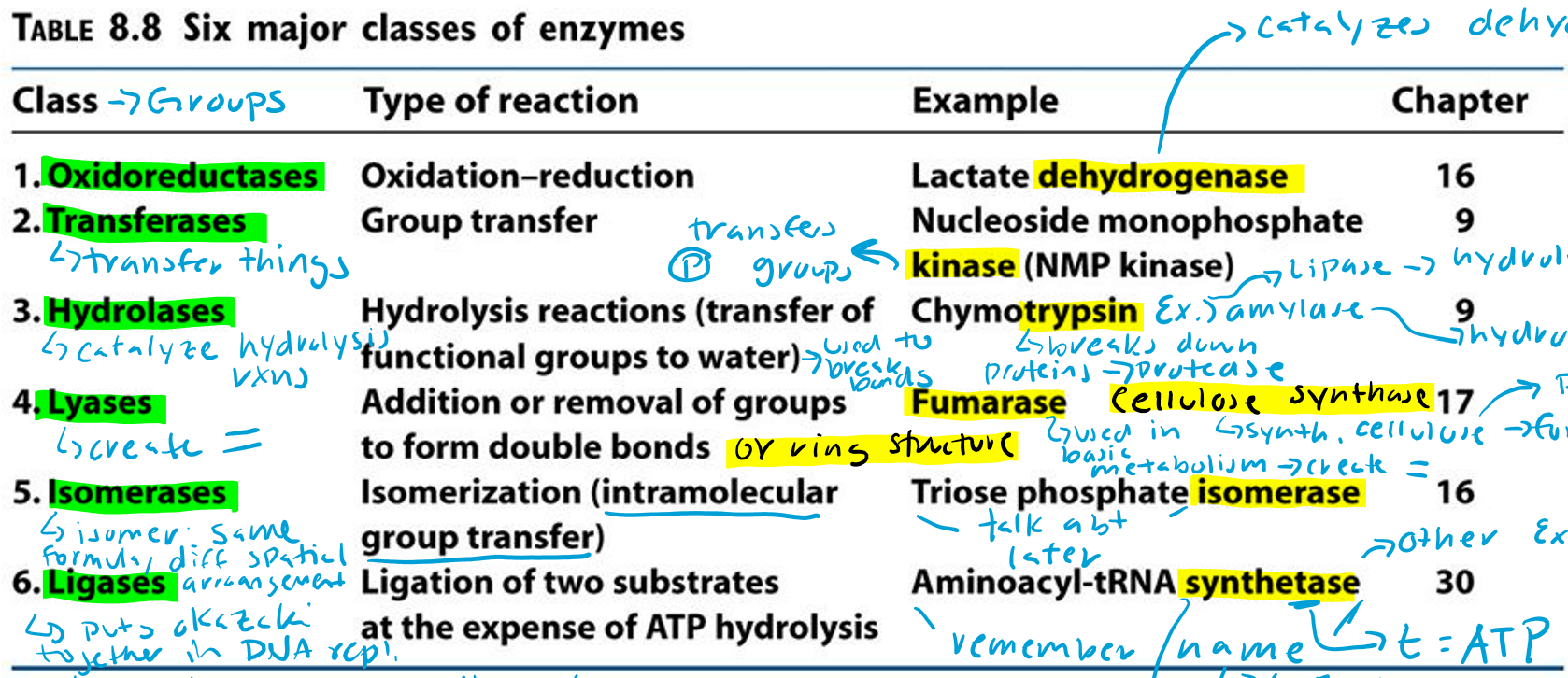
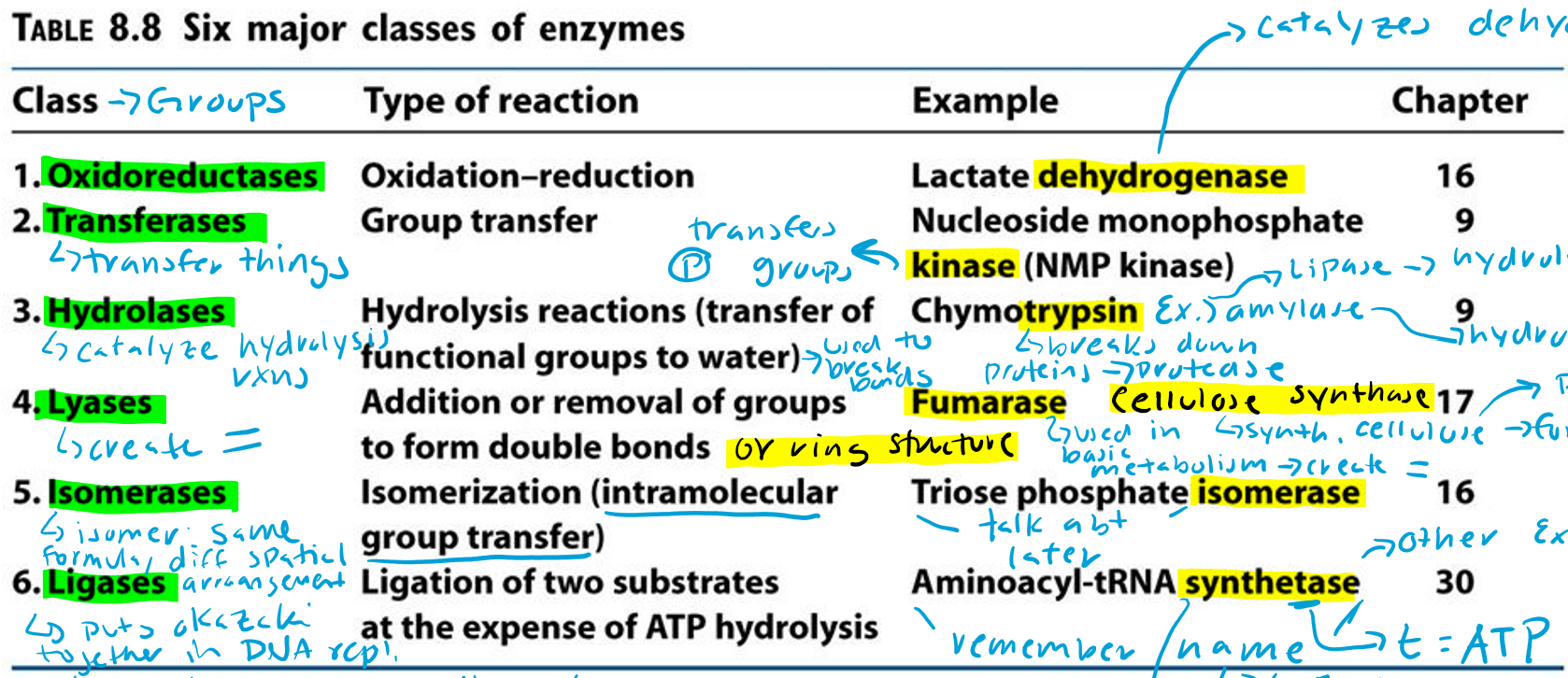
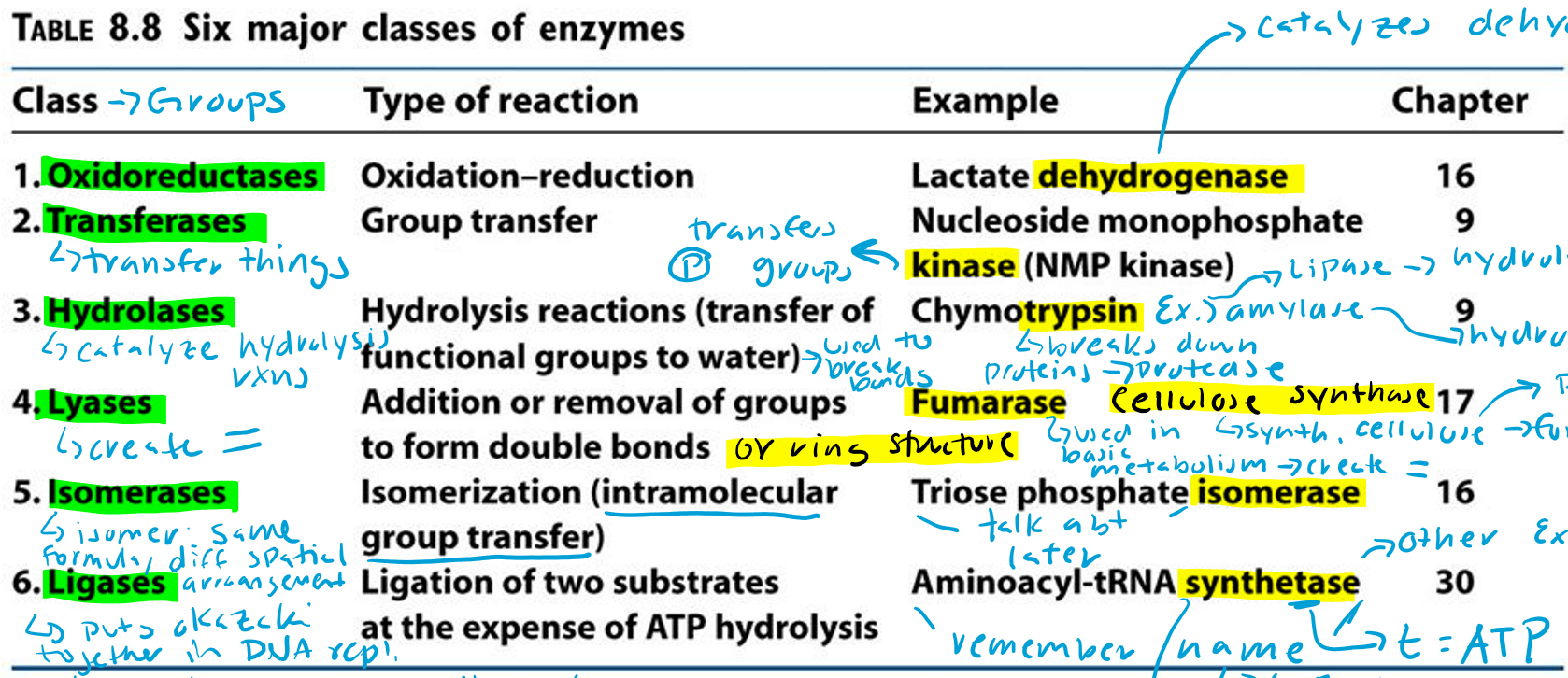
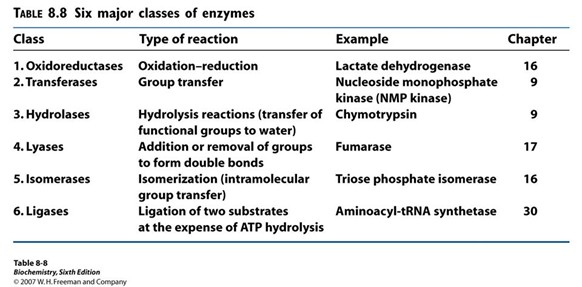
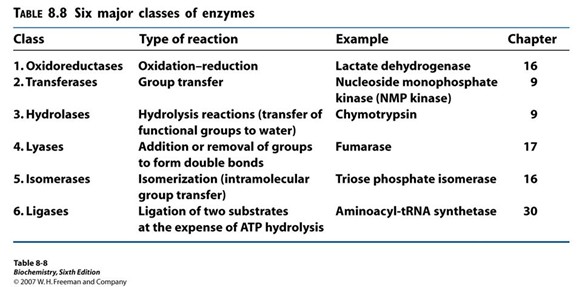
Oxidoreductases (Hint: 2)
Oxidation-reduction reaction
Example: Lactate dehydrogenase
Dehydrogenase (Hint: 3)
Catalyzes dehydrogenation
Remove H/e-
Oxidation reaction
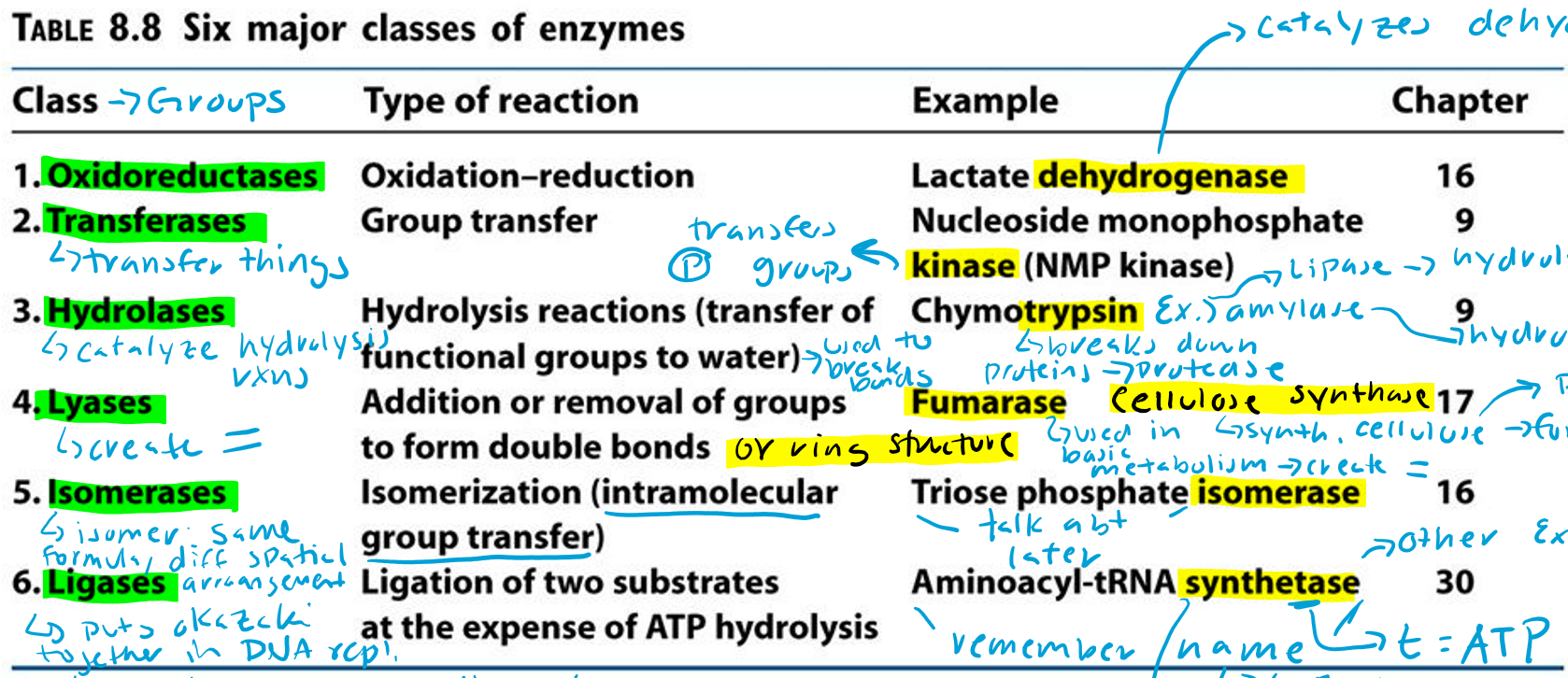
Transferases (Hint: 2)
Group transfer
Example: Nucleoside monophosphate kinase (NMP kinase)
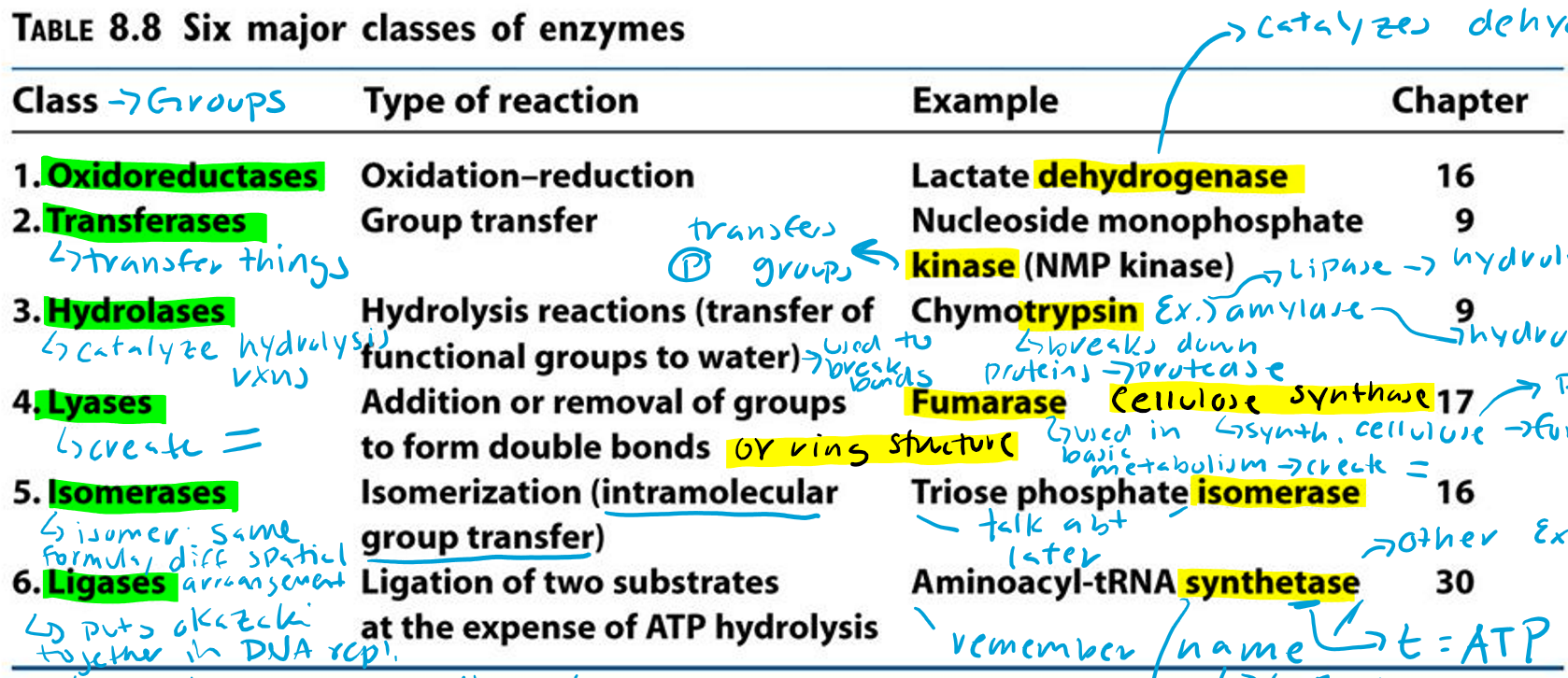
Kinase
Transfers phosphate groups
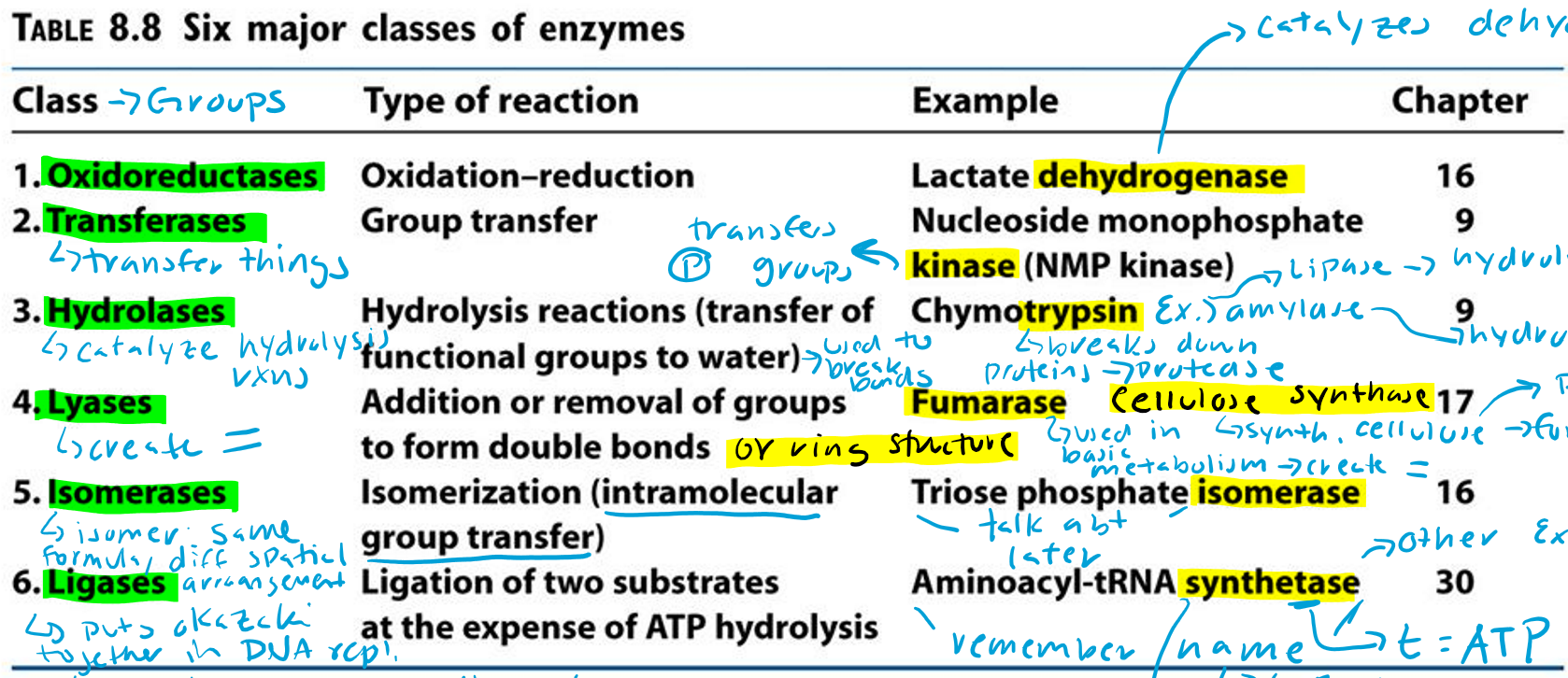
Hydrolases (Hint: 2)
Catalyze hydrolysis reactions (transfer of function groups to water)
Example: Chymotrypsin, Amylase and Lipase
Amylase
Hydrolyzes starch
Lipase
Hydrolyzes lipids
Trypsin
Breaks down proteins (protease)
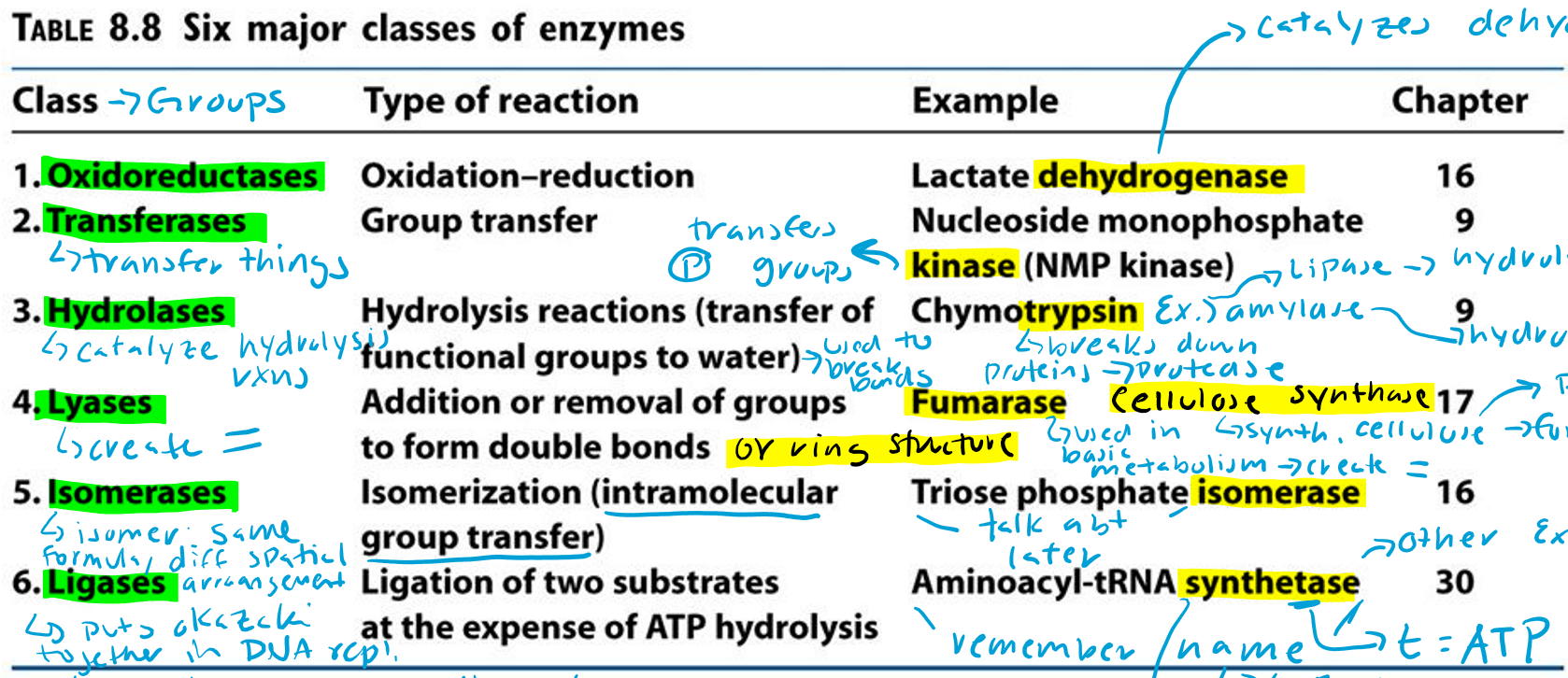
Lyases (Hint: 2)
Addition or removal of groups to form double bonds or ring structures
Example: Fumarase and Cellulose synthase
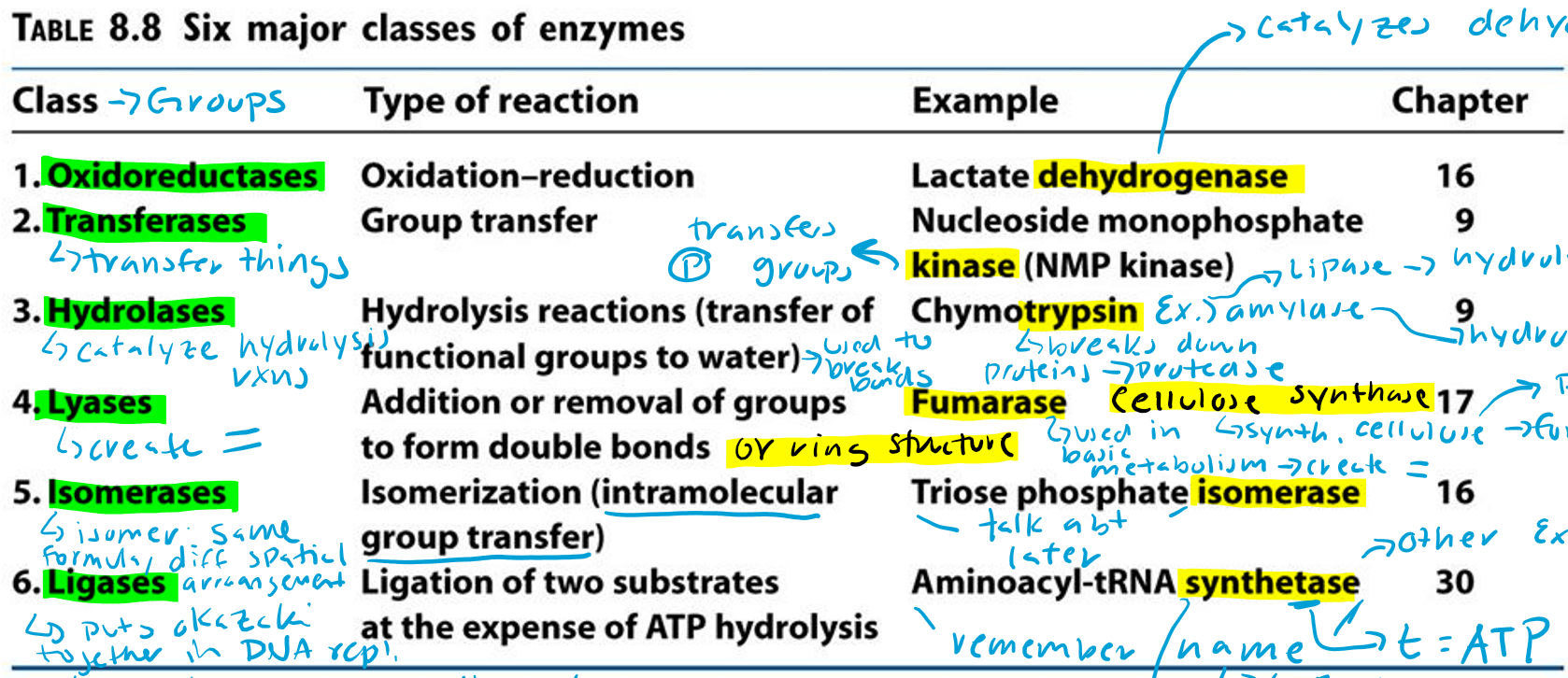
Fumarase (Hint: 2)
Used in basic metabolism
Creates double bonds
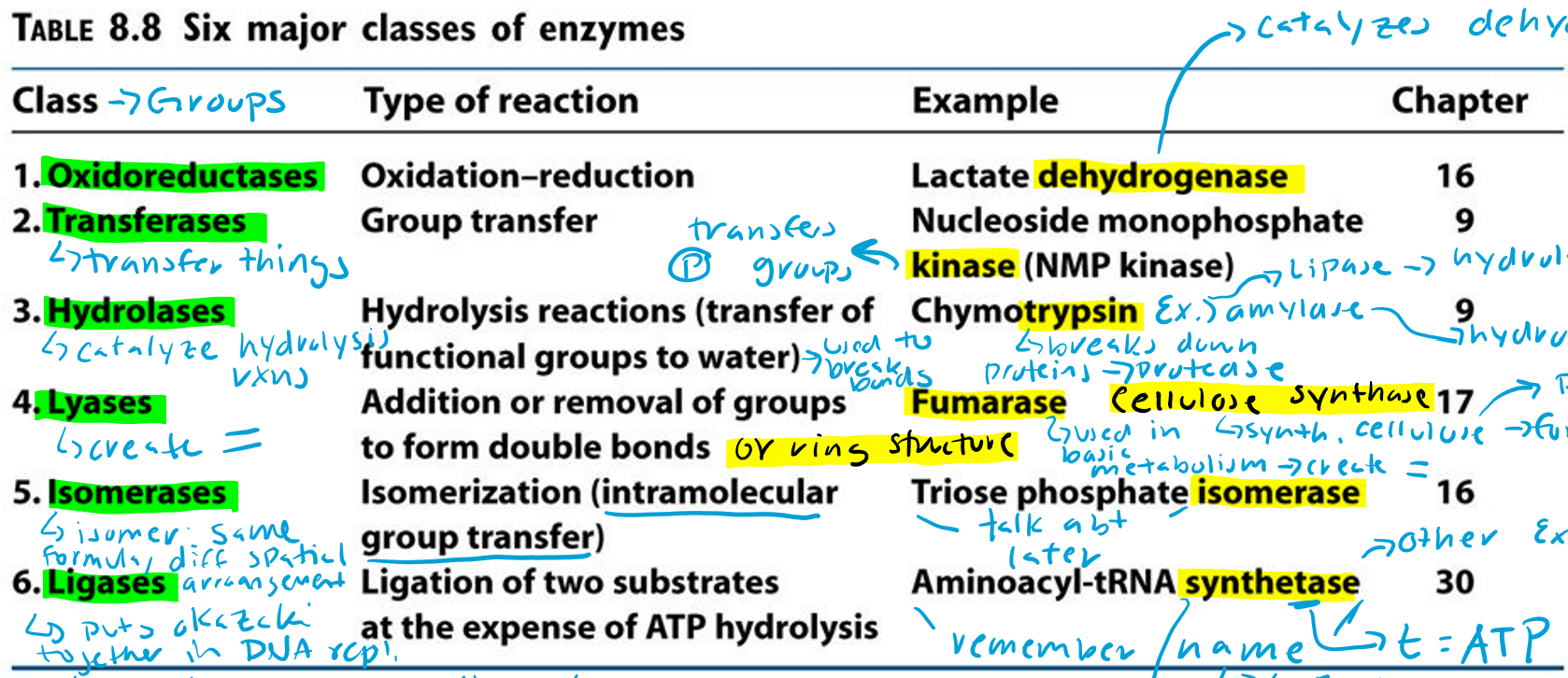
Cellulose Synthase (Hint: 2)
Synthesis of cellulose (polymer of glucose)
Forms ring structures
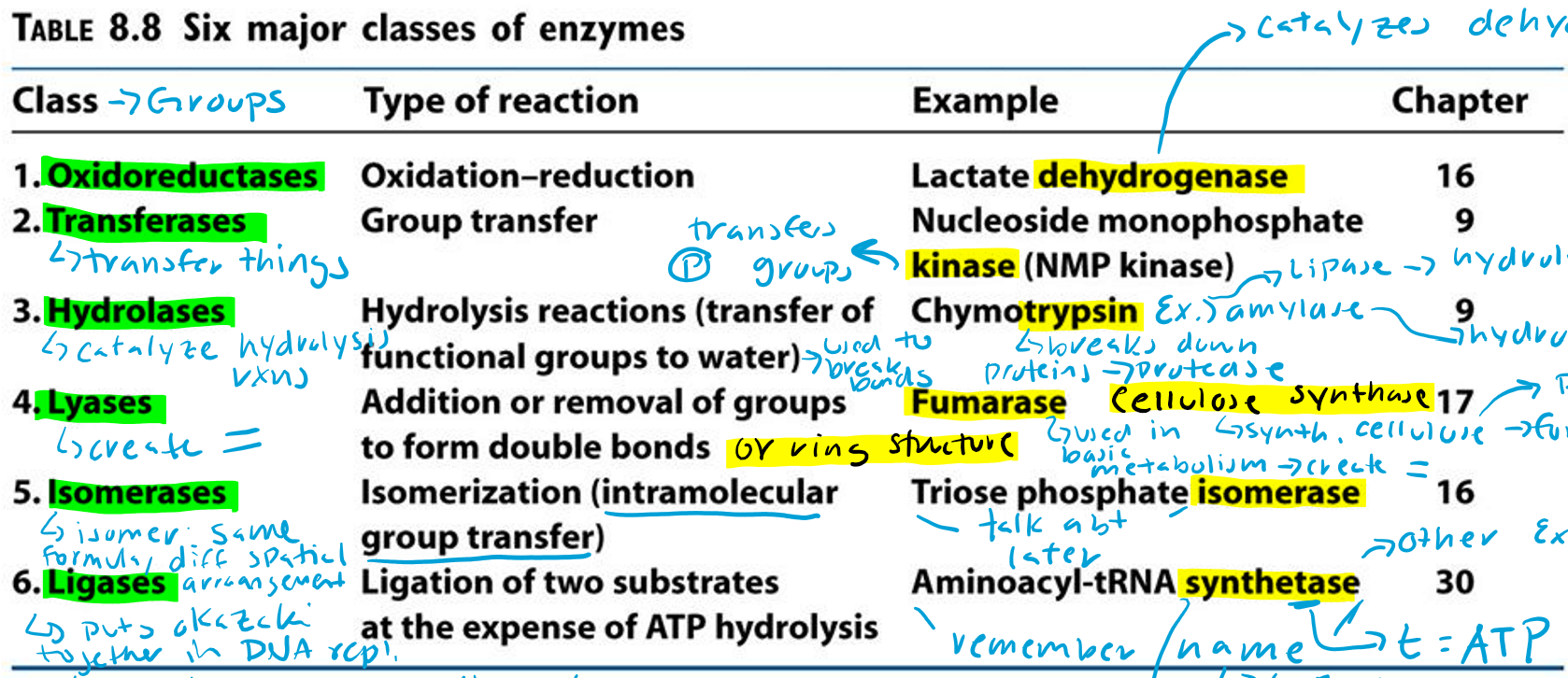
Isomerases (Hint: 2)
Isomerization (intramolecular group transfer)
Example: Triose Phosphate Isomerase
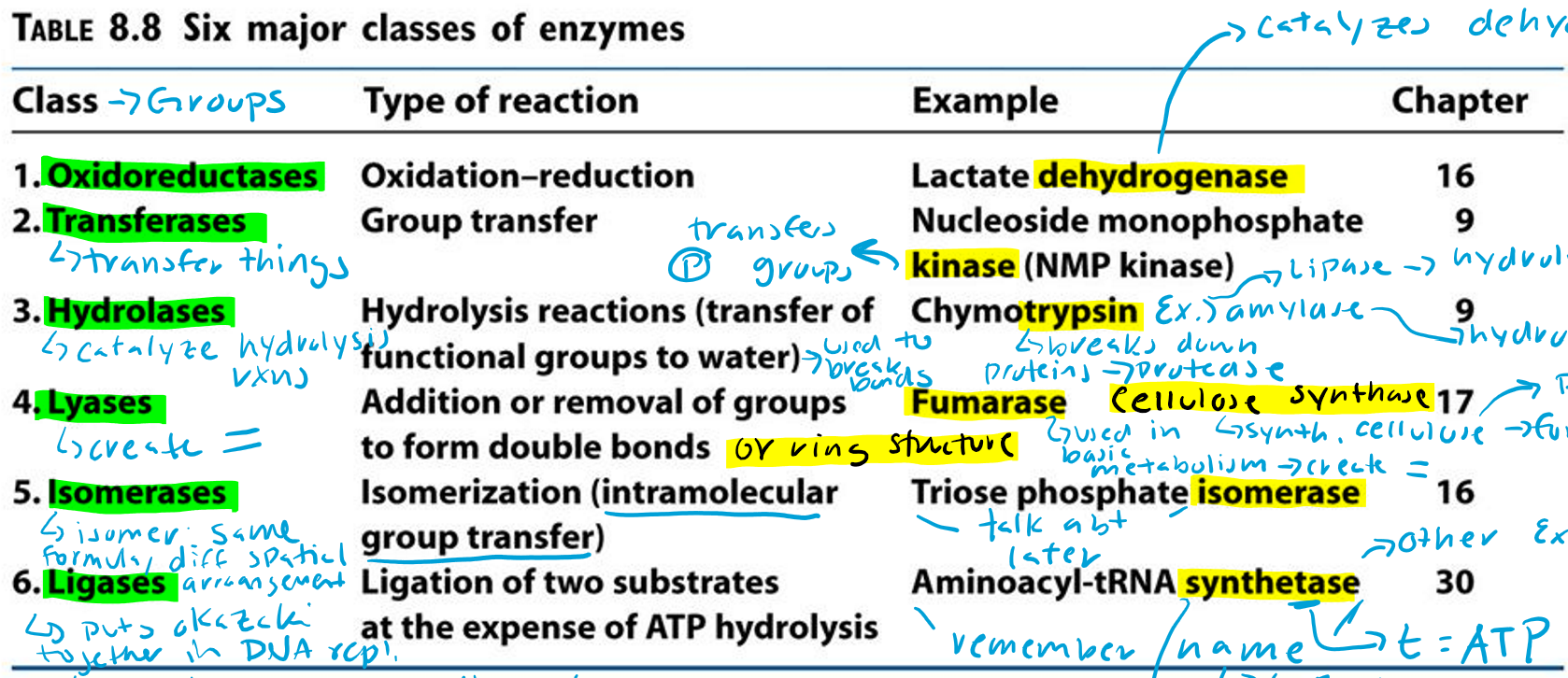
Isomer
Same formula, different spatial arrangement
Ligases (Hint: 4)
Ligation of two substrates at the expense of ATP Hydrolysis
Puts Okazaki fragments together in DNA replication
Make bonds to seal them together -> covalent bonds -> Uses energy from ATP
Example: Aminoacyl-tRNA Synthetase
Aminoacyl-tRNA Synthetase (Hint: 4)
t-RNA
Transfers amino acid to ribosome to make protein
Puts amino acid onto tRNA
Uses ATP to connect (no ATP = synthase)
Allosteric Control
Regulation of a protein by binding an effector molecule at a site other than the enzyme’s active site
Allosteric
Elsewhere in space
Allosteric Enzymes
Multi-subunit enzymes
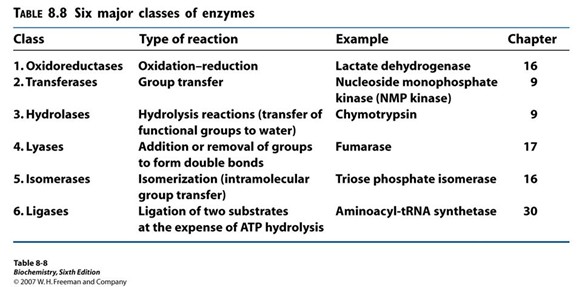
Aspartate Transcarbamoylase (ATCase)
Catalyzes the first step in pyrimidine synthesis
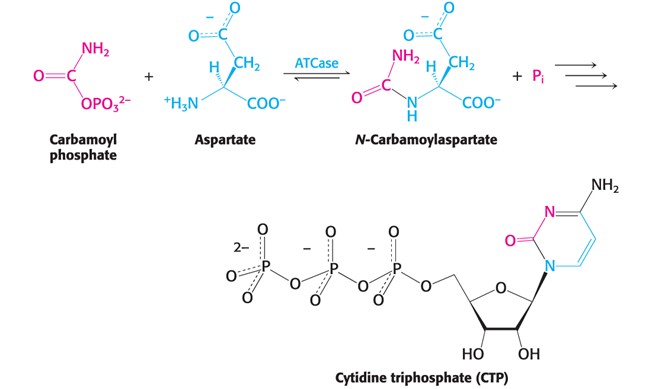
Sigmoidal Curve (Hint: 4)
Results from cooperativity between subunits/substrate-binding sites
Most allosteric enzymes have this type of kinetics
S-shaped “sigma”
Lag phase in the beginning
Homotropic Effects (Hint; 2)
The effects of substrates on allosteric enzymes
Similar movement effects
Concerted (“all or none”) mechanism for allosteric enzymes (Hint: 2)
All active sites are in the same state, either T or R
In one enzyme, if 1 picks up substrate, all pick up substrate
Cooperativity
All subunits need to work together
Catalytic Subunit (Hint: 2)
Catalyze reaction
Bind to substrate
Regulatory Subunit (Hint: 2)
Control enzyme activity
Allosterically binds
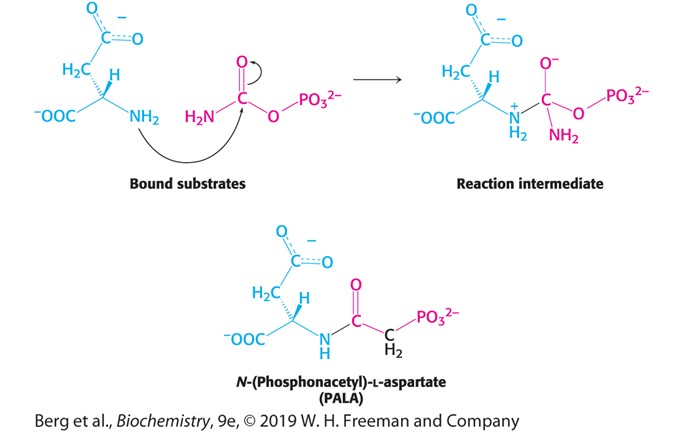
Bi-Substrate Analog (Hint: 2)
Molecule that looks like 2 substrates
Come up with molecules that looks like reaction intermediate
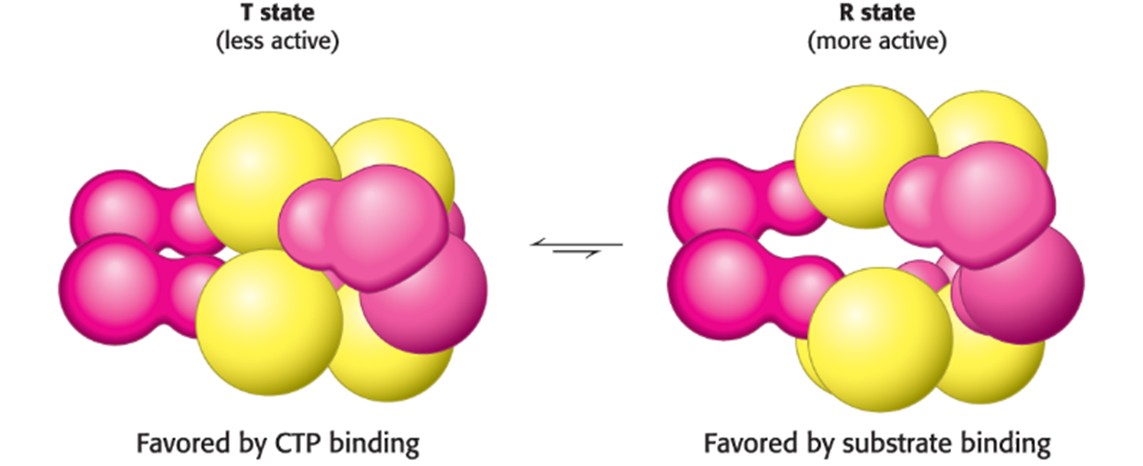
T-State (Hint: 4)
Tensed
More compact
Less functional
Removal of substrate/addition of allosteric inhibitor make T-state
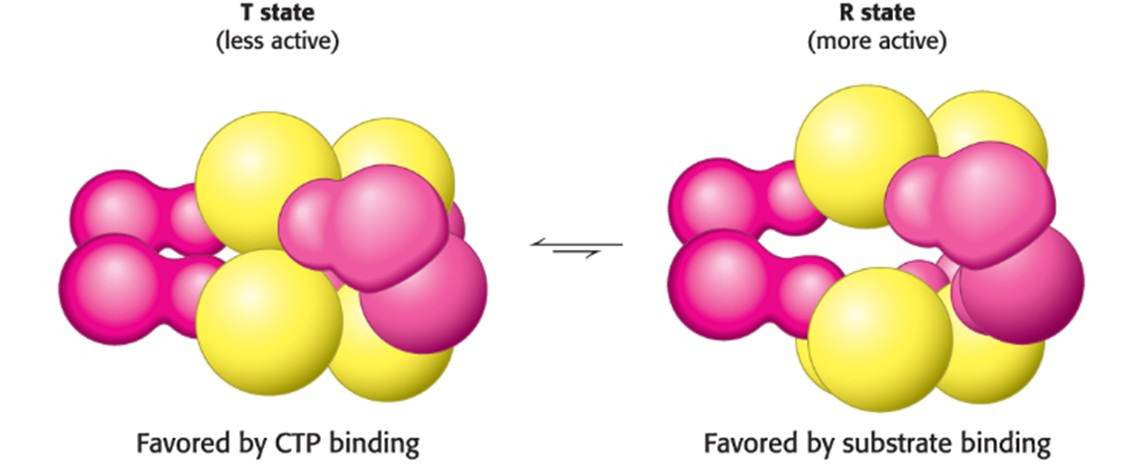
R-State (Hint: 3)
Relaxed
More functional
Substrate binding makes it relaxed
List the 6 major classes of enzymes
Oxidoreductases
Transferases
Hydrolases
Lyases
Isomerases
Ligases
Enzymatic Activity is Regulated in what 4 Principal Ways?
Allosteric control: Controlled by molecule that is binding somewhere other than active site
Multiple forms of enzymes: Use different versions of same enzyme
Reversible covalent modification: Phosphorylation and acetylation; Add covalent bond and change shape/activity
Proteolytic activation: Some need to be digested before it can work
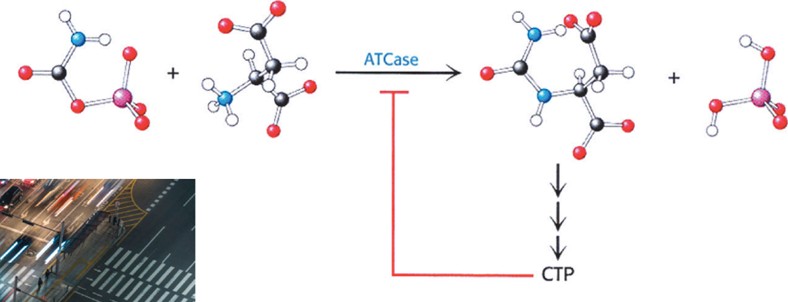
Describe ATCase Reaction (Hint: 4)
Allosterically inhibited by the end product of its pathway, CTP, in an example of feedback inhibition
If high CTP, it binds to ATCase, changes shape, and stops enzyme
CTP exerts its effects by binding at a distinct regulatory or allosteric site on ATCase
Displays Sigmoidal Kinetics and does not display Michaelis Menton Kinetics
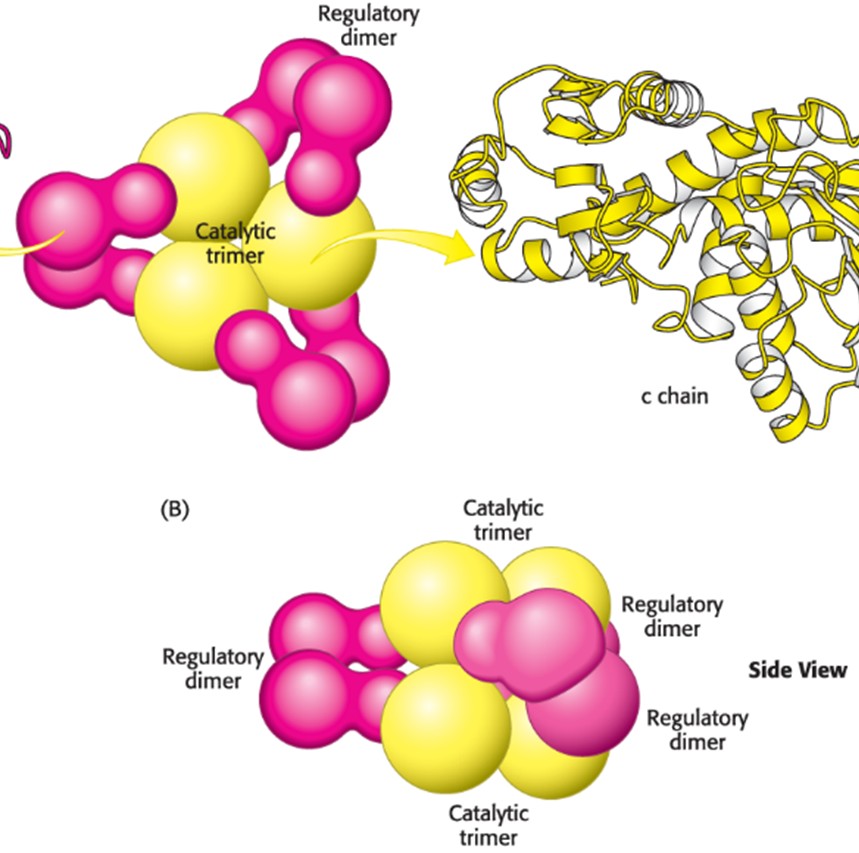
Describe the ATCase Active Site (Hint: 5)
Consists of 6 catalytic and 6 regulatory subunits (c6r6)
Behaves like one protein (enzyme)
Active sites are located at the interface of the catalytic subunits
Each catalytic subunit has its own active site
Identified by use of the bi-substrate analog PALA
Describe PALA
Binding of PALA (substrate) causes structural changes that convert the compact, less active T state into the expanded, active R state
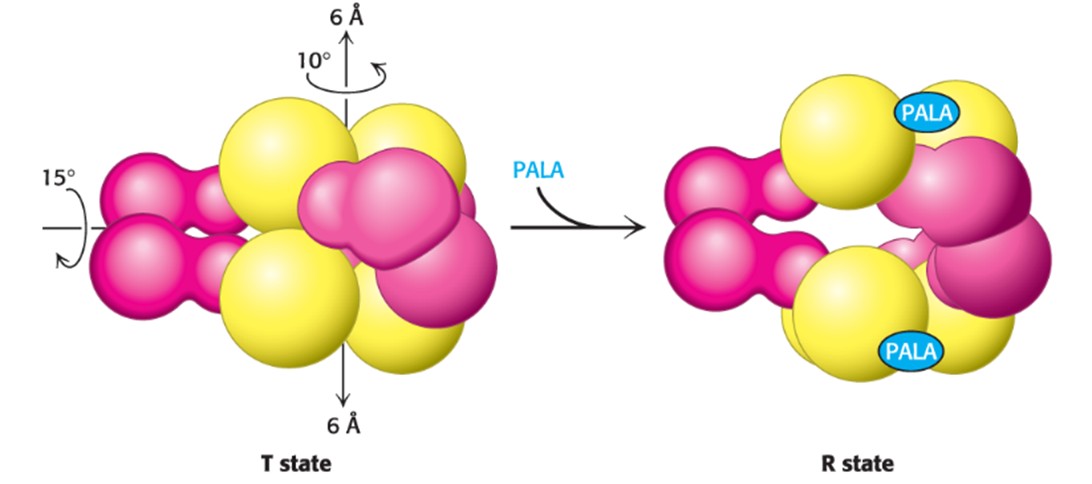
Describe the T and R State in terms of ATCase (Hint: 4)
The T state has a low affinity for substrate and has low catalytic activity, while the R state is the most active form
The two states are in equilibrium, with the T state being favored in the absence of substrate and in the presence of CTP
Binding of substrate disrupts the equilibrium in favor of the R state, this involves cooperativity (when substrate starts binding to catalytic trimer, all 3 subunits are activated)
Binding of CTP to the regulatory sire of ATCase alters the T-to-R equilibrium in favor of the T state, decreasing net enzyme activity (more CTP = more T-state enzymes)
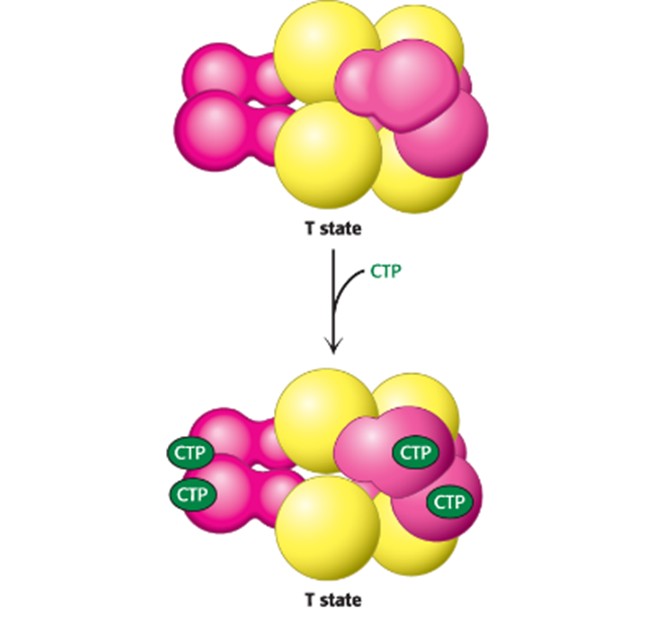
What mediates allosteric interactions in ATCase? (Hint: 2)
Large changes in Quaternary structure
If there is allosteric effect of ATCase (CTP), large scale changes of structure of enzyme
The sigmoidal kinetic curve of allosteric enzymes allows what?
Increased sensitivity to changes in substrate concentration
ATCase follows what mechanism?
Concerted (“all or none”) mechanism