ATI TEAS 7: Basic Atomic Structure & Bonding Flashcards
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
Atom
Smallest unit of an element that retains its properties

Proton
Positively charged particle found in the nucleus

Neutron
Neutral particle found in the nucleus

Electron
Negatively charged particle found in the electron cloud

Nucleus
Center of the atom containing protons and neutrons
Atomic Number
Number of protons in an atom

Mass Number
Total number of protons and neutrons

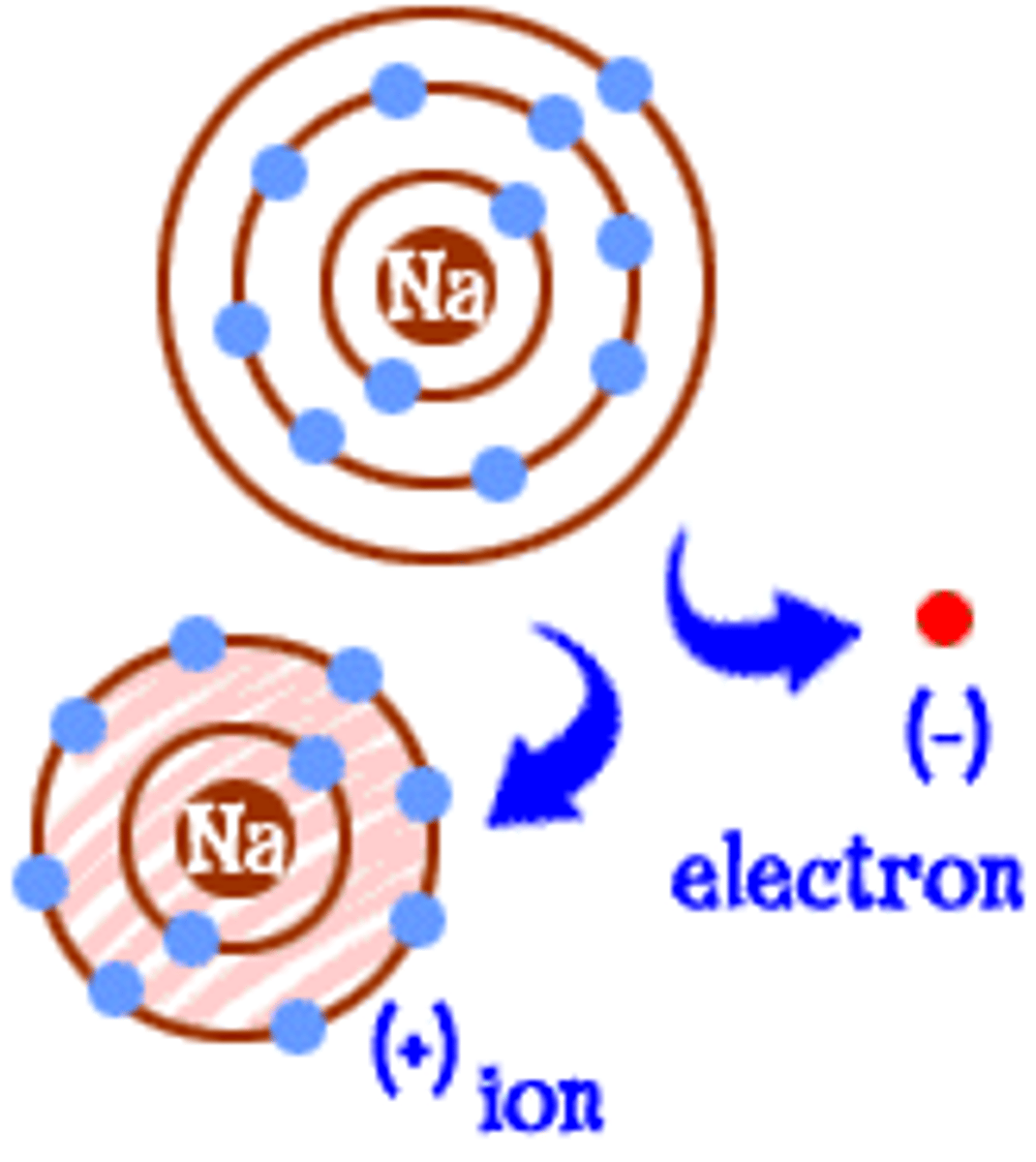
Ion
Atom that has gained or lost electrons
Cation
Positively charged ion formed by losing electrons

Anion
Negatively charged ion formed by gaining electrons
What changes when an ion forms?
Number of electrons
What never changes when an ion forms?
Number of protons

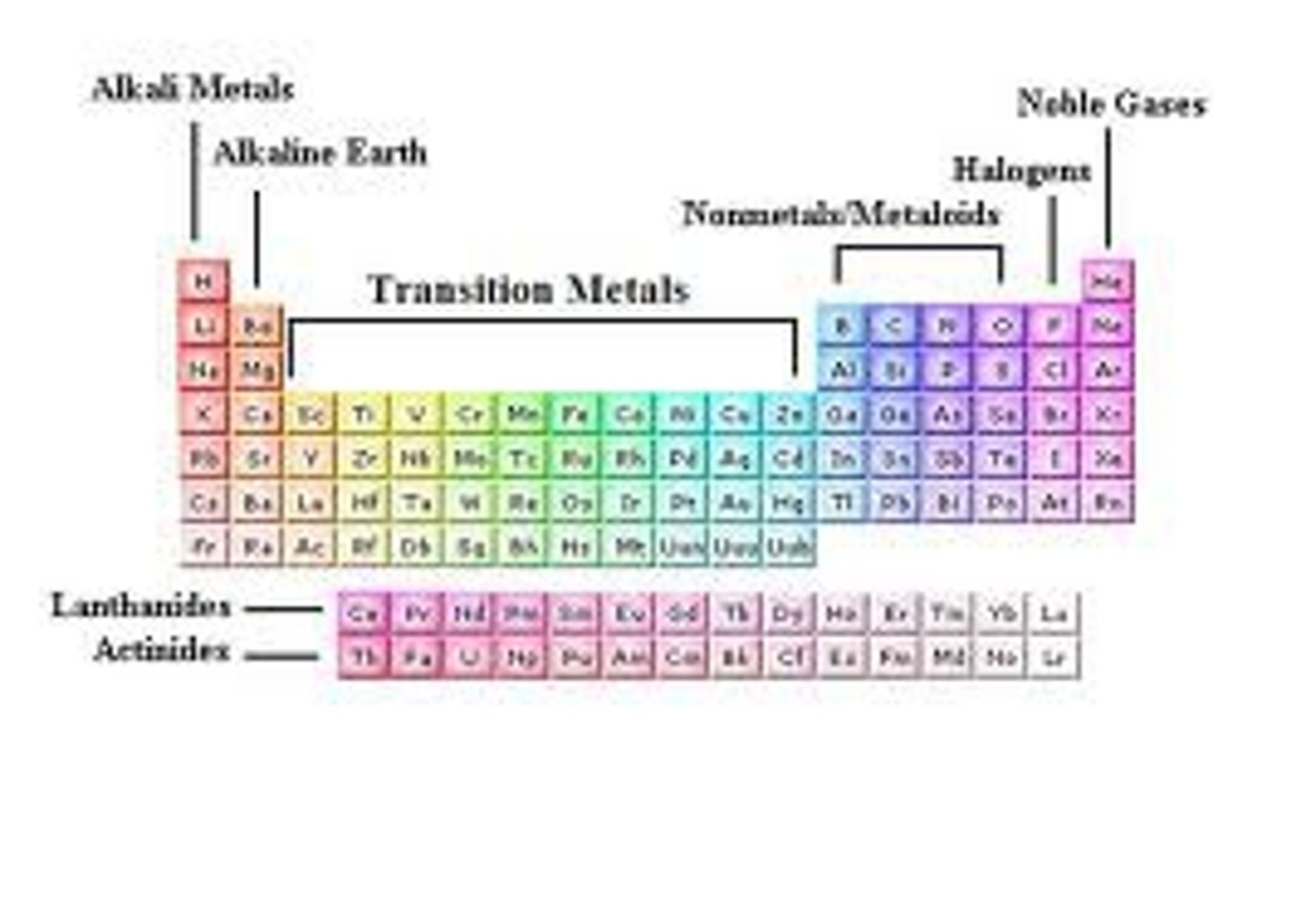
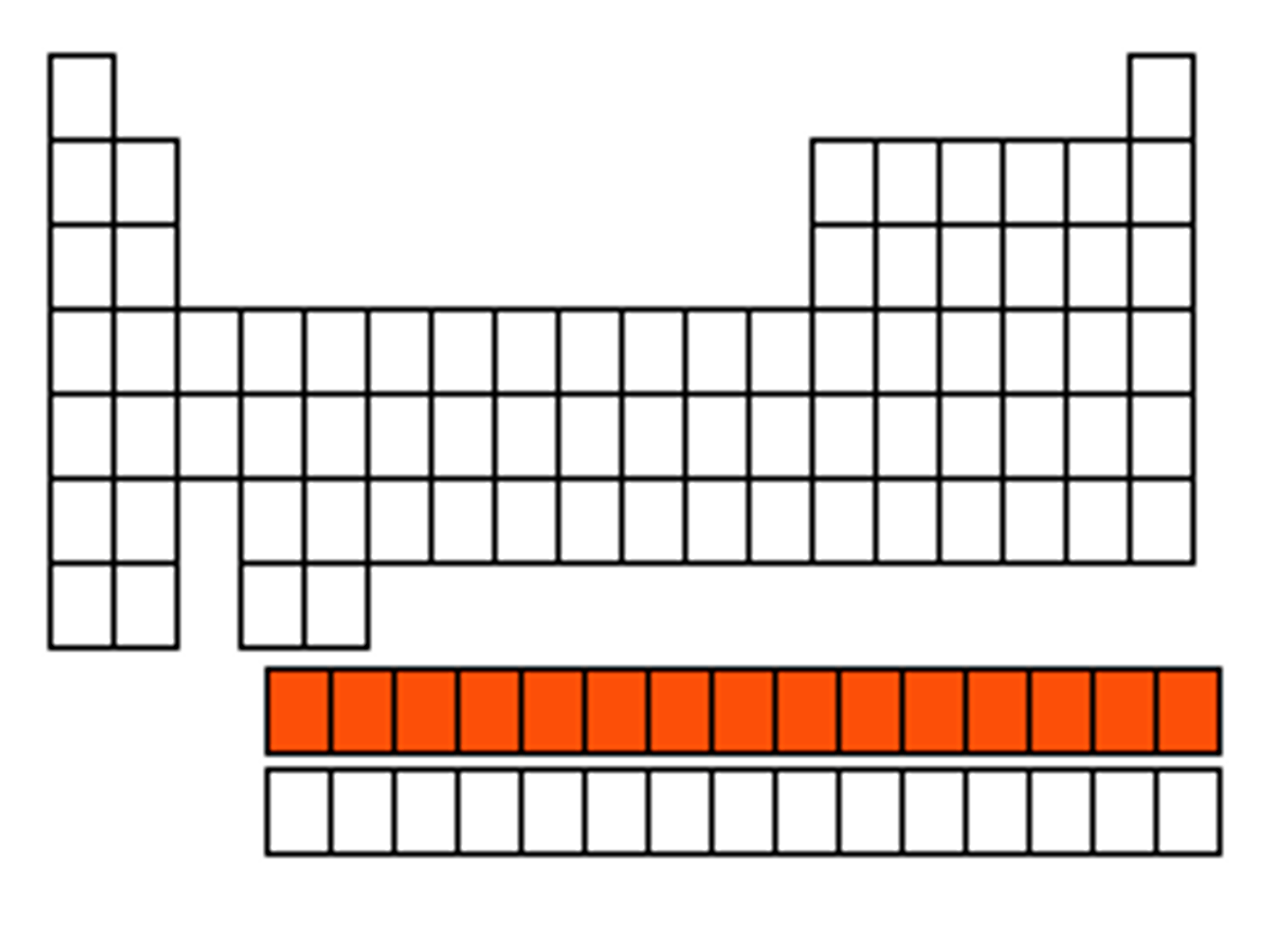
Periodic Table
Chart that organizes elements by atomic number
Group (Periodic Table)
Vertical column; elements with similar properties
Period (Periodic Table)
Horizontal row; indicates energy levels
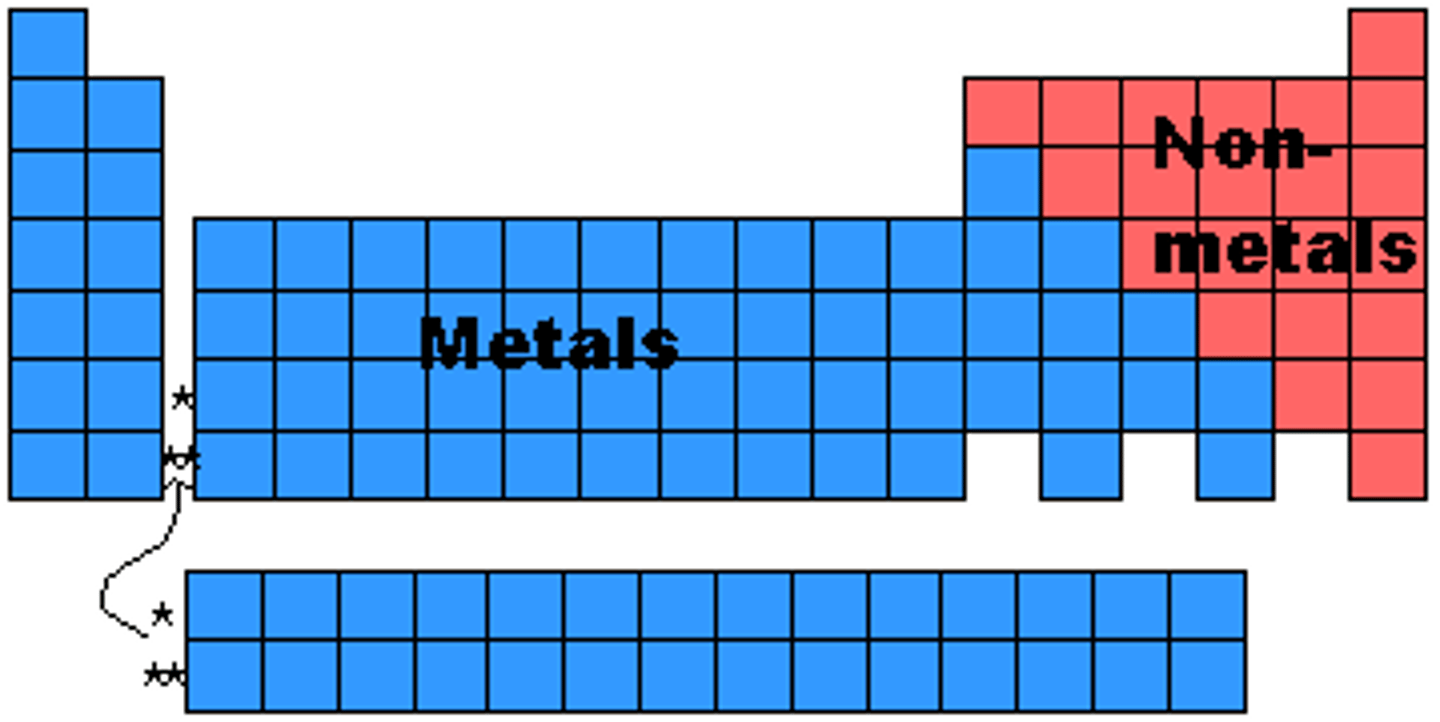
Metals (Periodic Table)
Tend to lose electrons and form cations
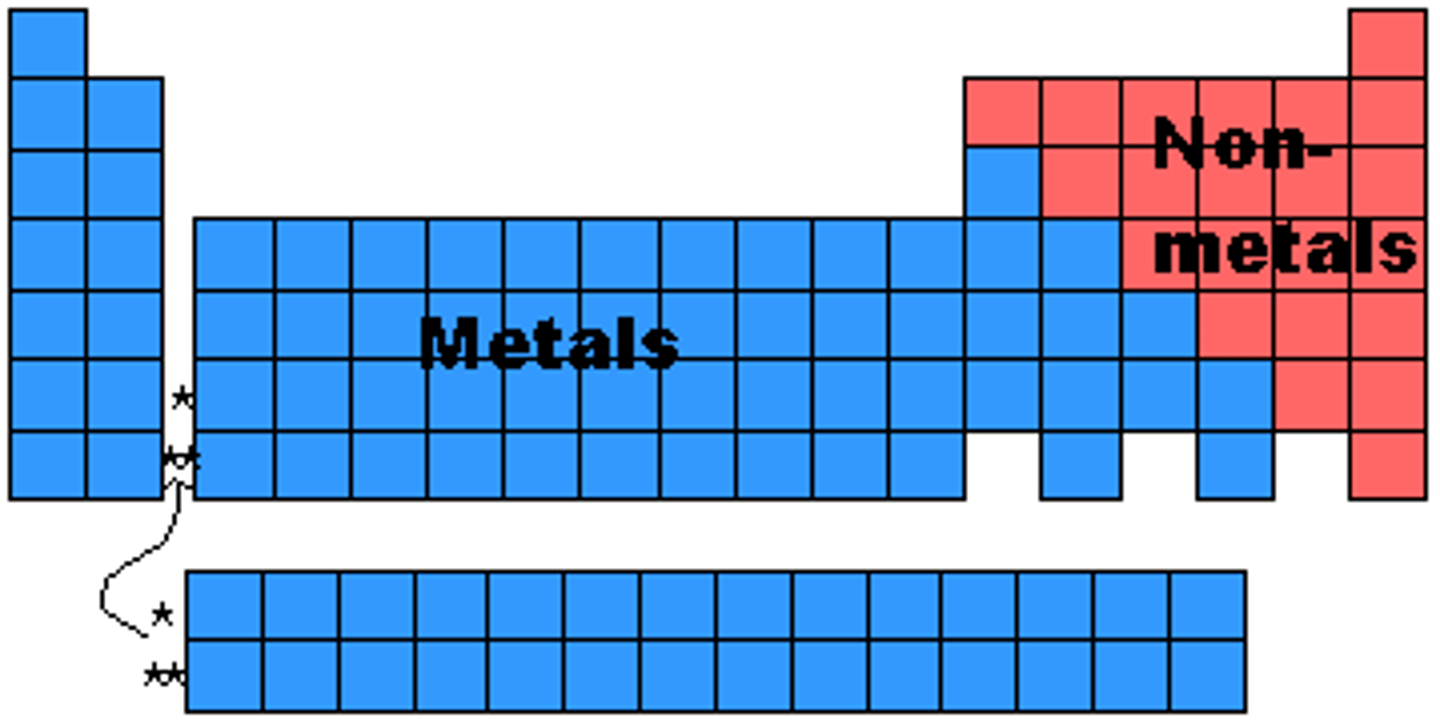
Nonmetals (Periodic Table)
Tend to gain electrons and form anions
Noble Gases
Stable elements that rarely react
Covalent Bond
Chemical bond formed by sharing electrons
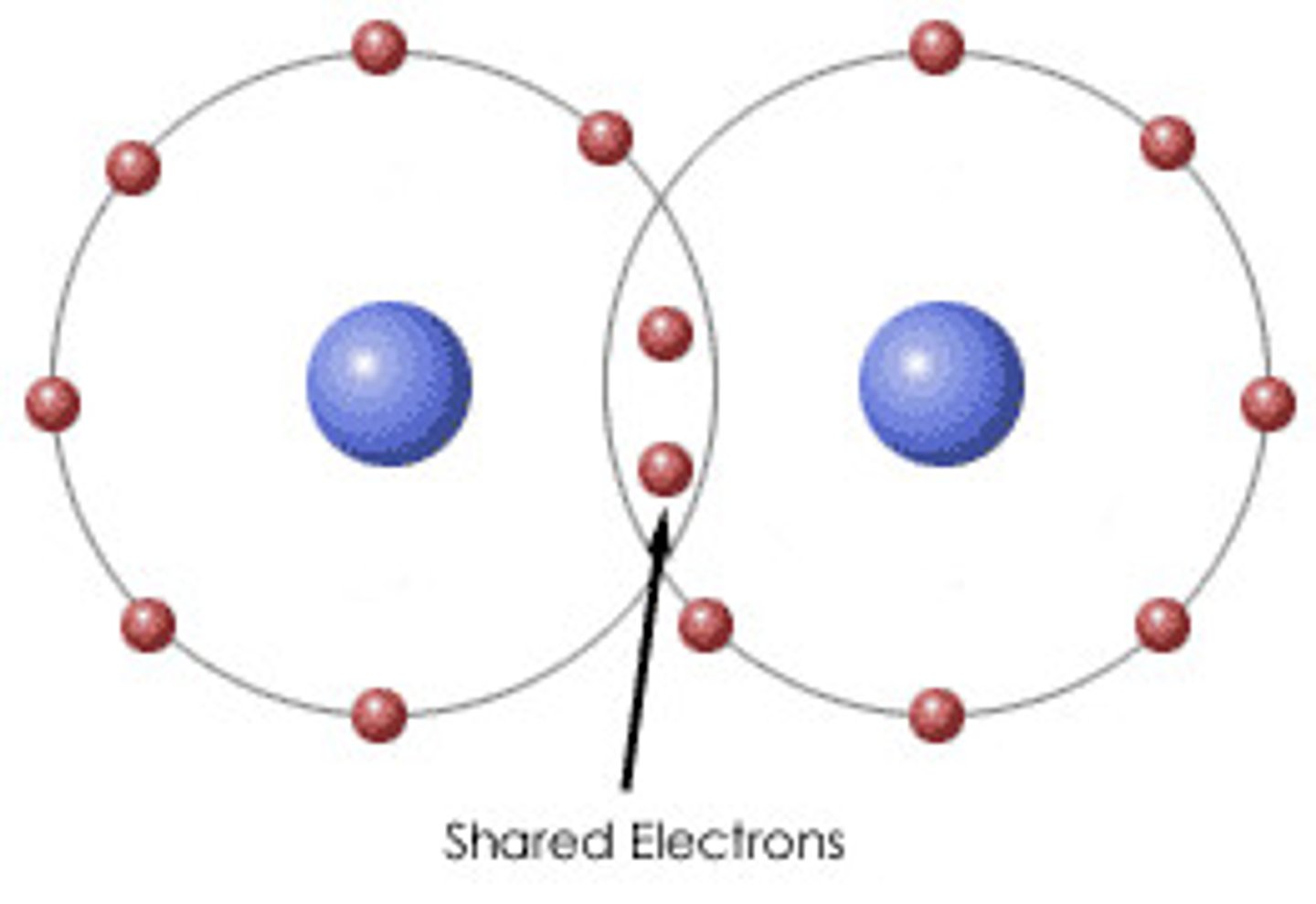
Covalent Bond Forms
Molecules
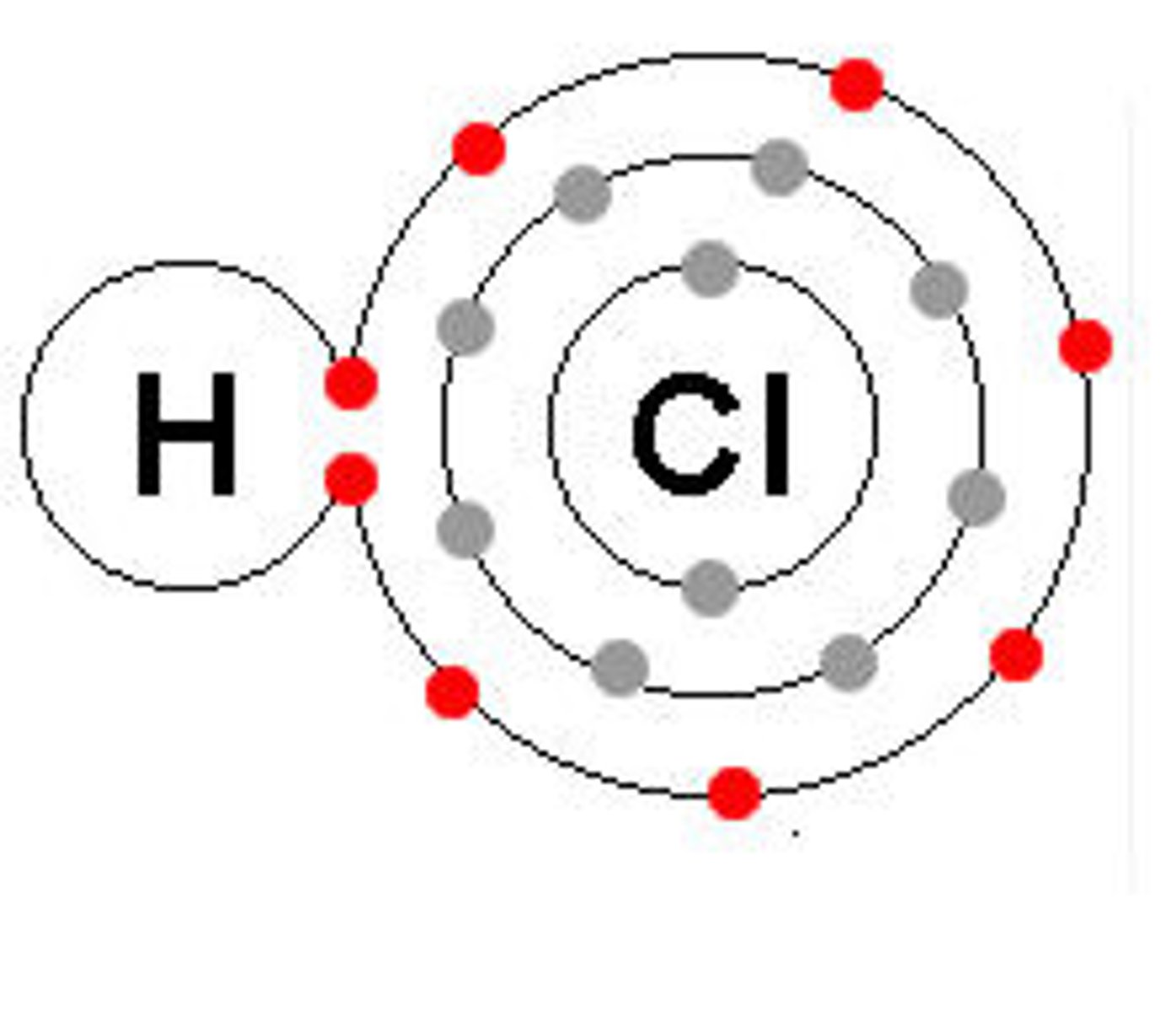
Covalent Bond Example
H₂O
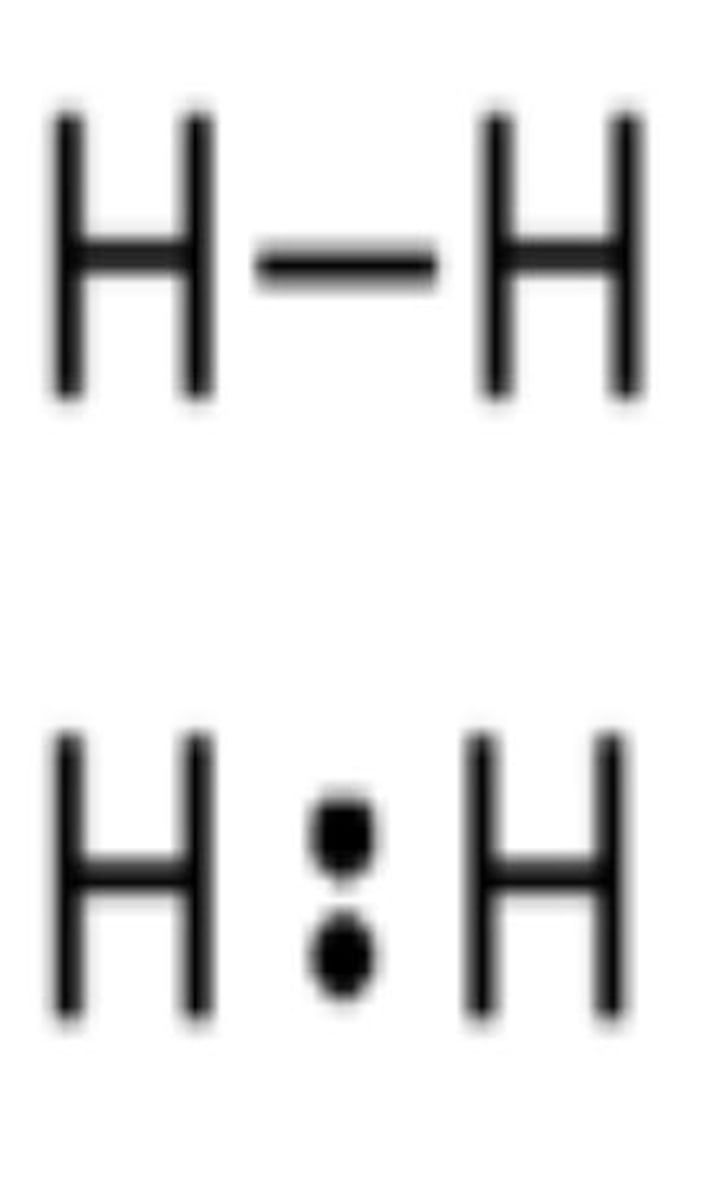
Ionic Bond
Chemical bond formed by transfer of electrons

Ionic Bond Forms
Ions and ionic compounds
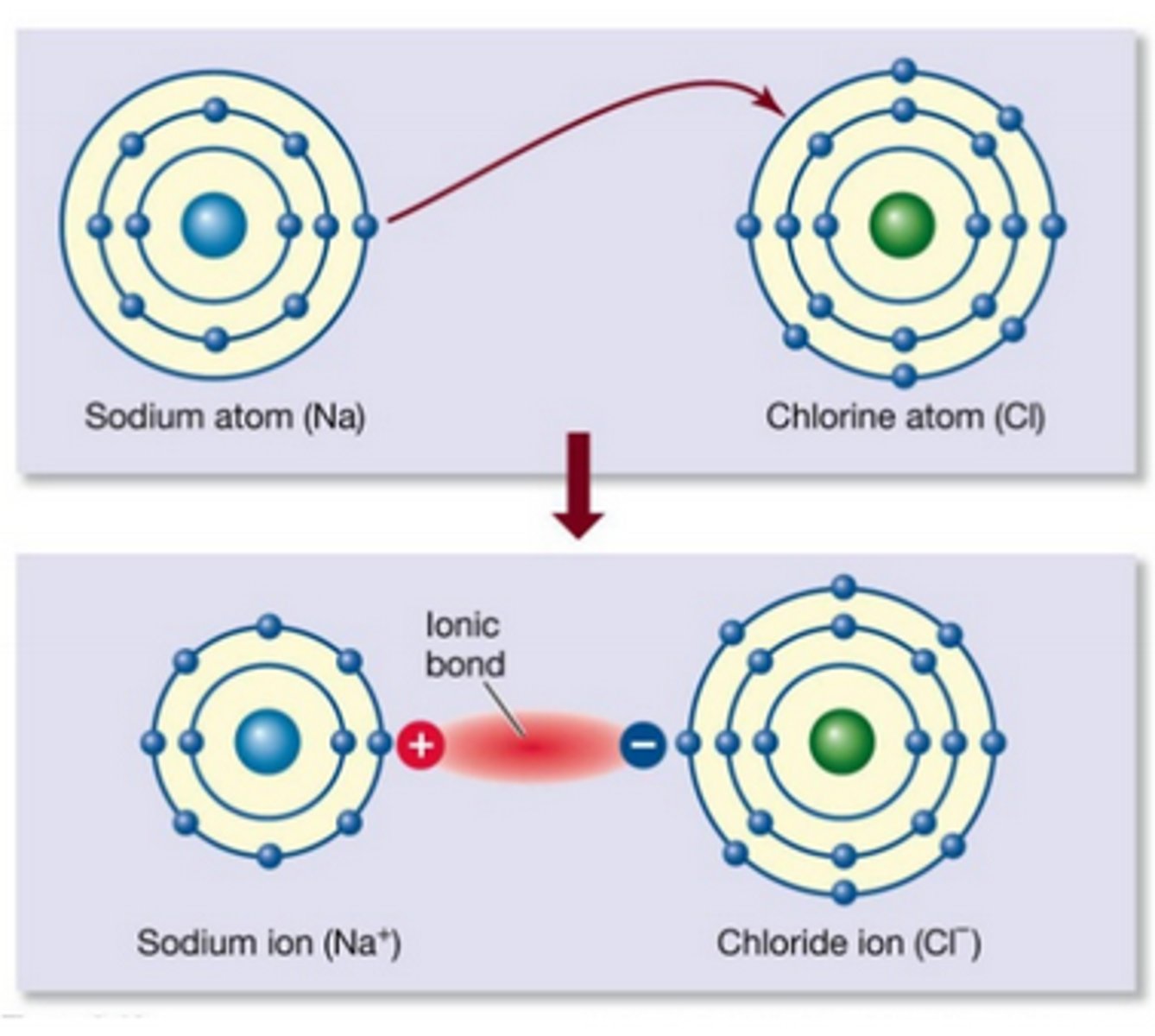
Ionic Bond Example
NaCl
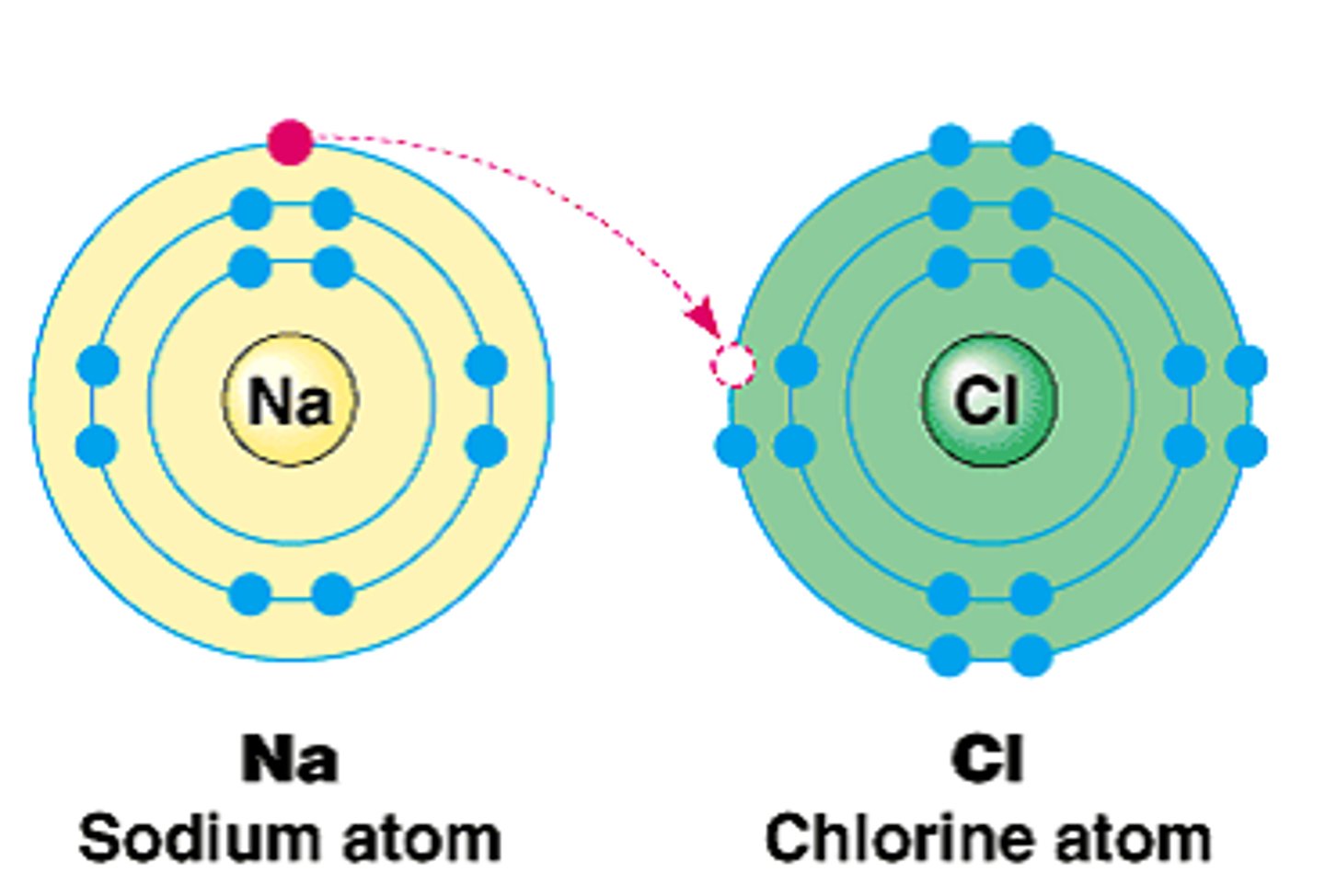
Which bond is stronger: ionic or covalent?
Ionic bond
Which bond involves metals and nonmetals?
Ionic bond
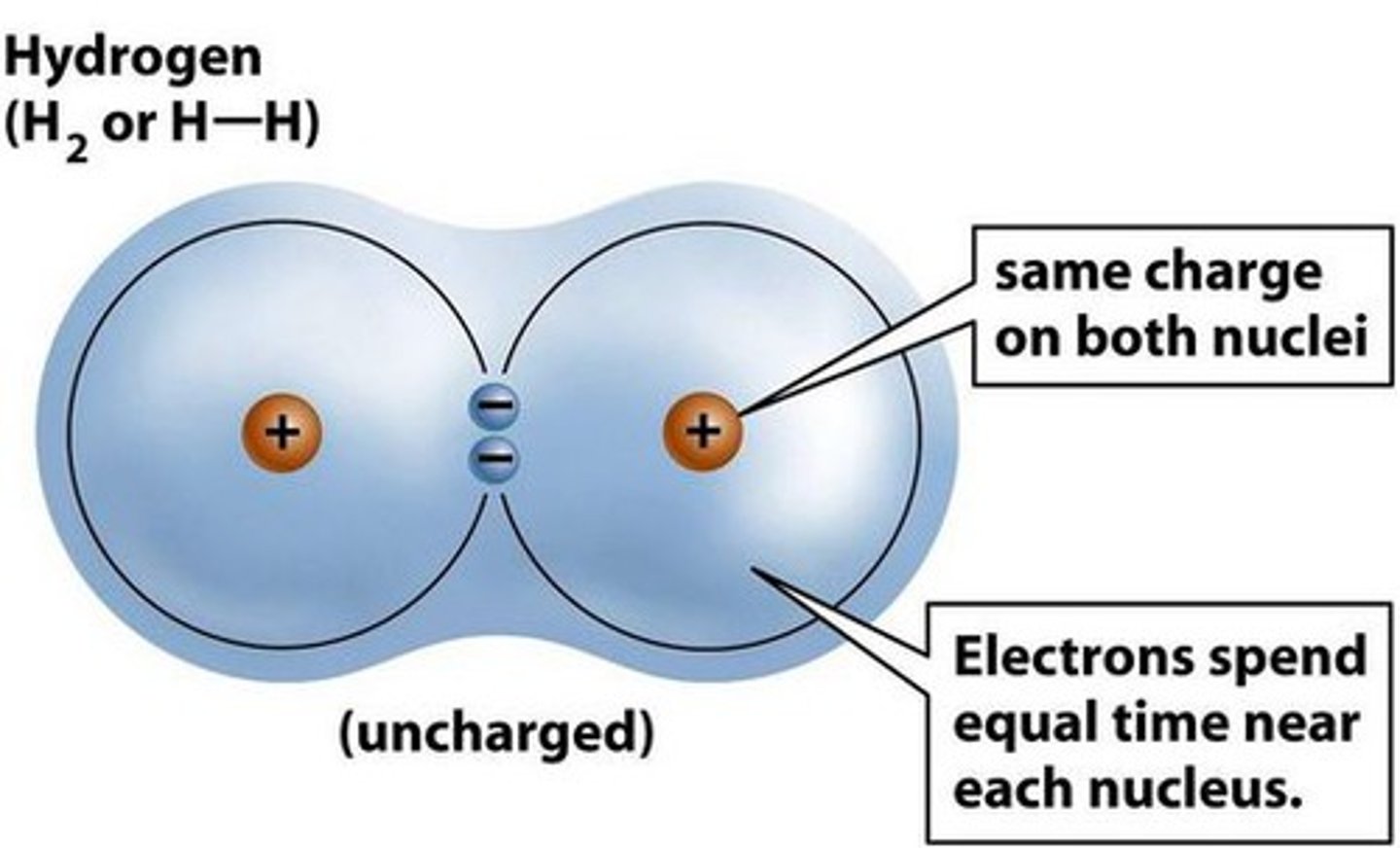
Which bond involves only nonmetals?
Covalent bond
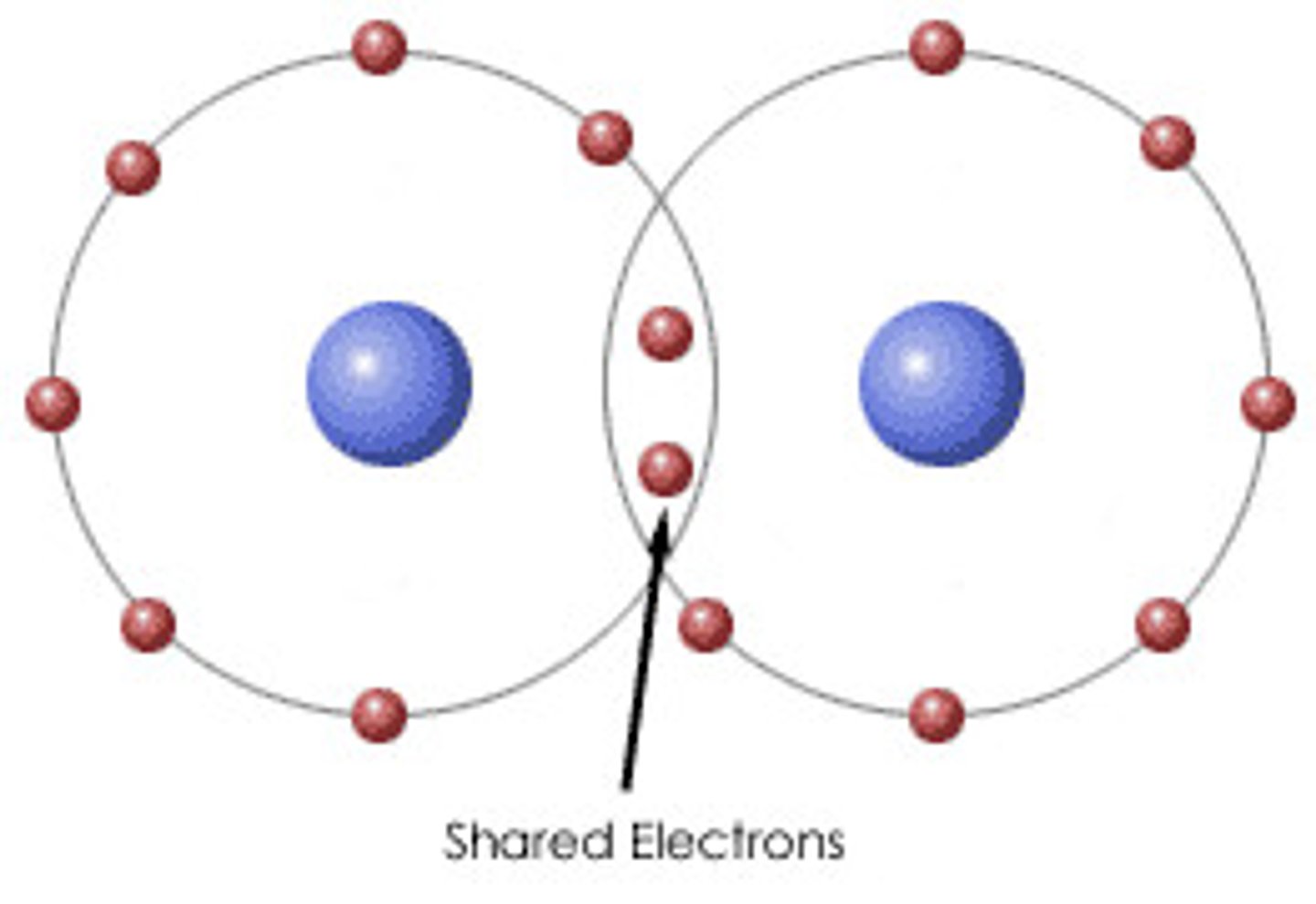
TEAS Memory Tip - Protons
Identify the element
TEAS Memory Tip - Electrons
Determine bonding and ion formation
TEAS Memory Tip - Neutrons
Add mass to the atom
Diagram shows center with + symbols
Nucleus containing protons
Diagram shows neutral particles in nucleus
Neutrons
Diagram shows small dots orbiting nucleus
Electrons
Diagram labels center of atom
Nucleus
Diagram shows atom with equal protons and electrons
Neutral atom
Diagram shows more electrons than protons
Anion (negative ion)
Diagram shows fewer electrons than protons
Cation (positive ion)
Diagram shows atomic number highlighted
Number of protons
Diagram shows mass number highlighted
Protons + neutrons
Diagram shows electron shells/energy levels
Regions where electrons are found
Diagram shows outermost shell emphasized
Valence electrons
Diagram shows electron transfer from one atom to another
Ionic bond
Diagram shows electrons shared between atoms
Covalent bond
Diagram shows metal giving electron to nonmetal
Ionic compound formation
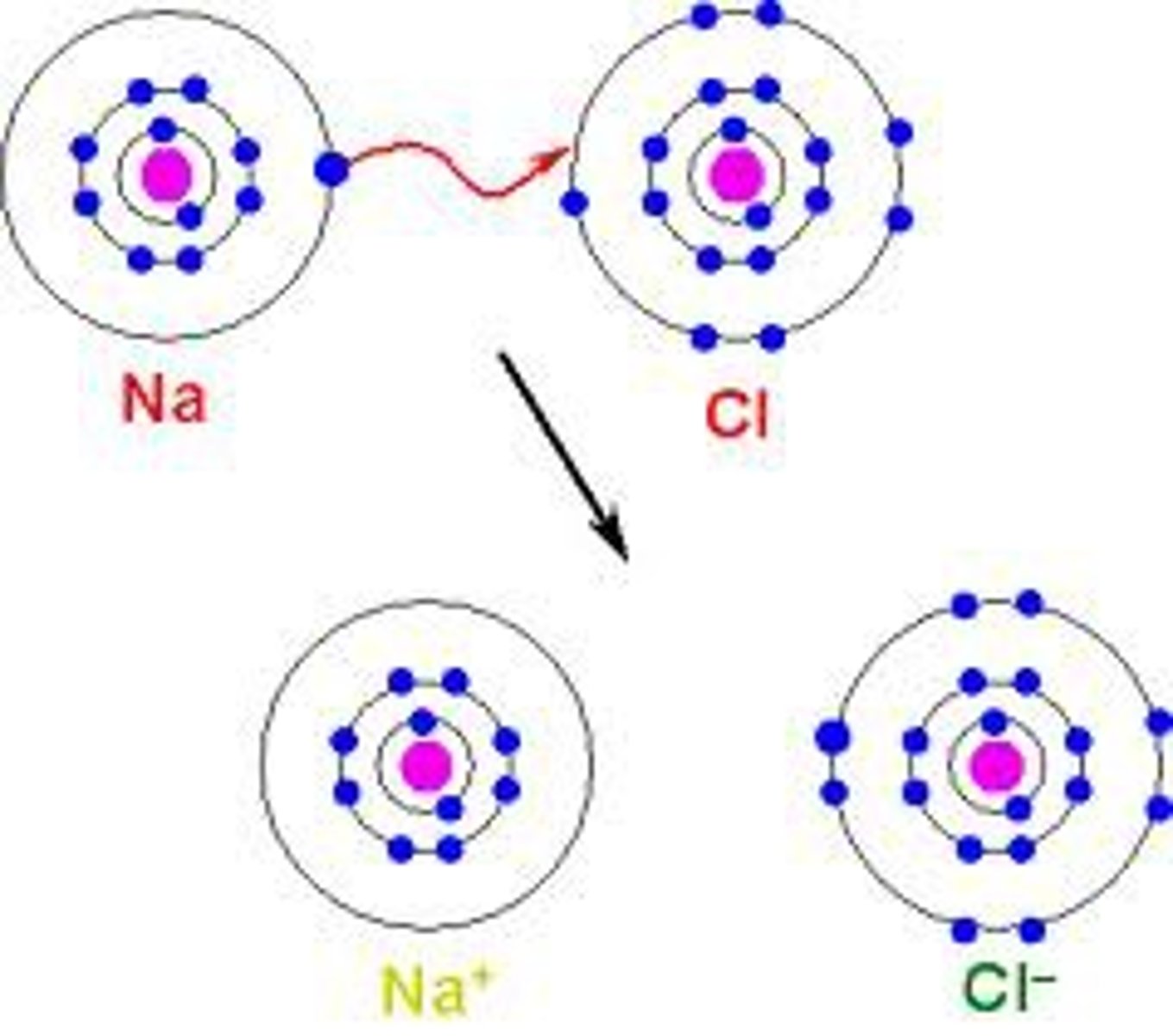
Diagram shows overlapping electron clouds
Covalent molecule
Diagram of Na losing one electron
Sodium ion (Na⁺)
Diagram of Cl gaining one electron
Chloride ion (Cl⁻)

Diagram shows brackets with charge symbol
Ion notation
Diagram shows periodic table with group highlighted
Elements with similar properties
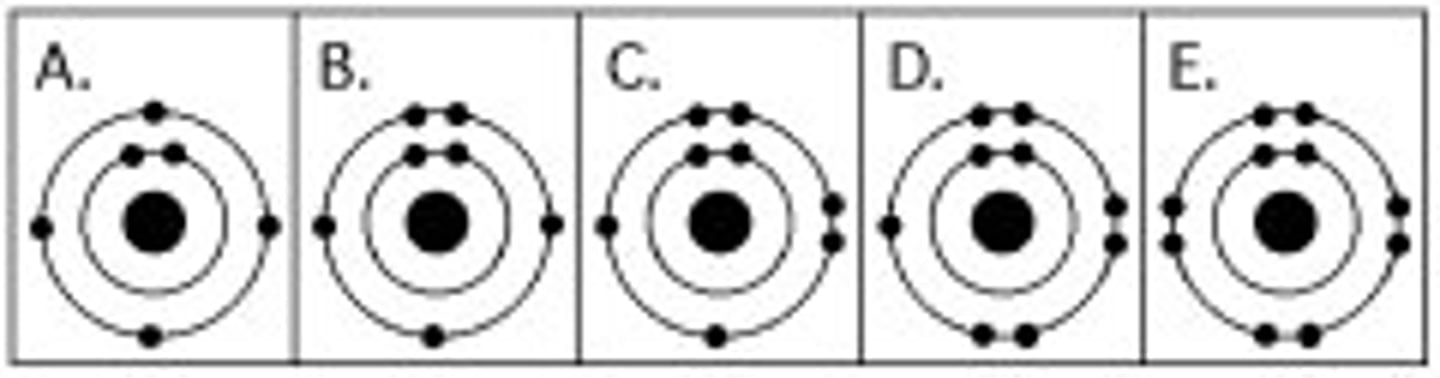
Diagram shows periodic table row highlighted
Period (energy level)
+ in nucleus =
Protons
Orbiting dots =
Electrons
Extra electrons =
Negative ion
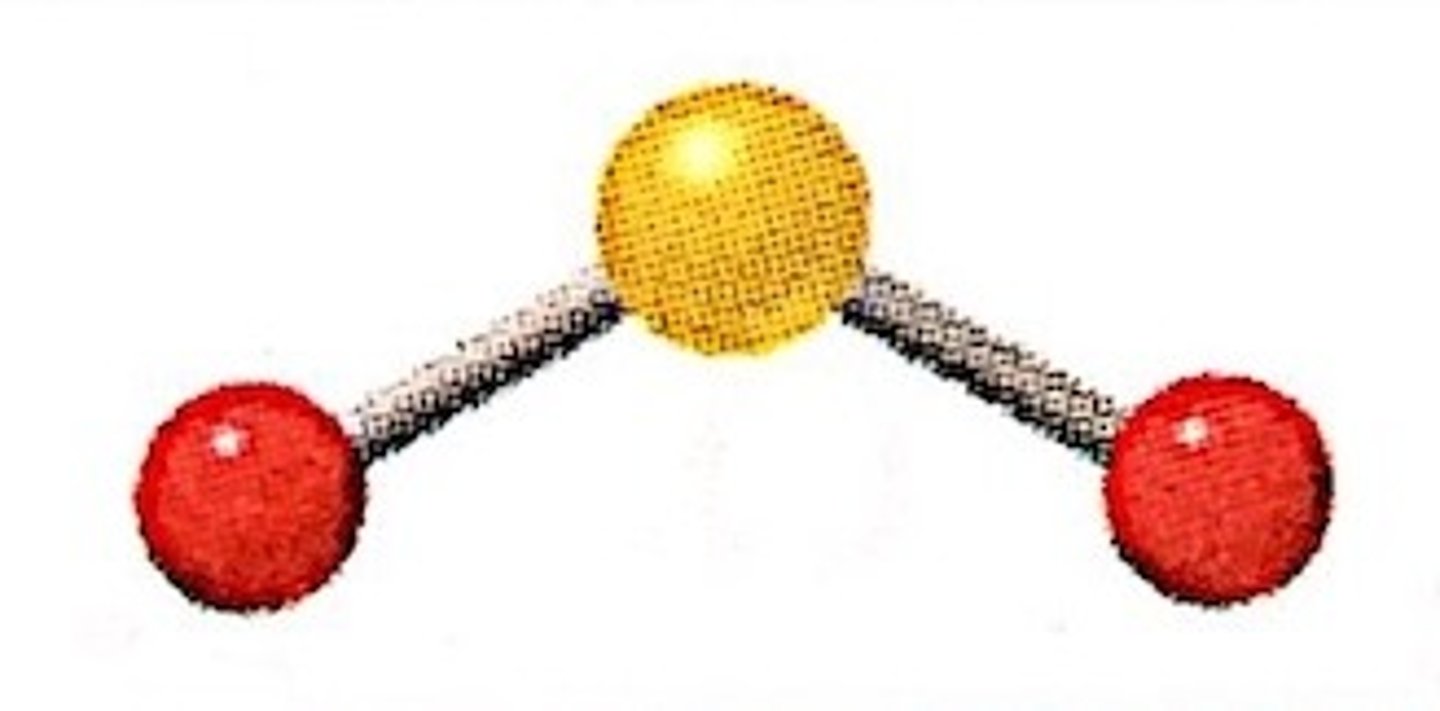
Shared dots =
Covalent bond
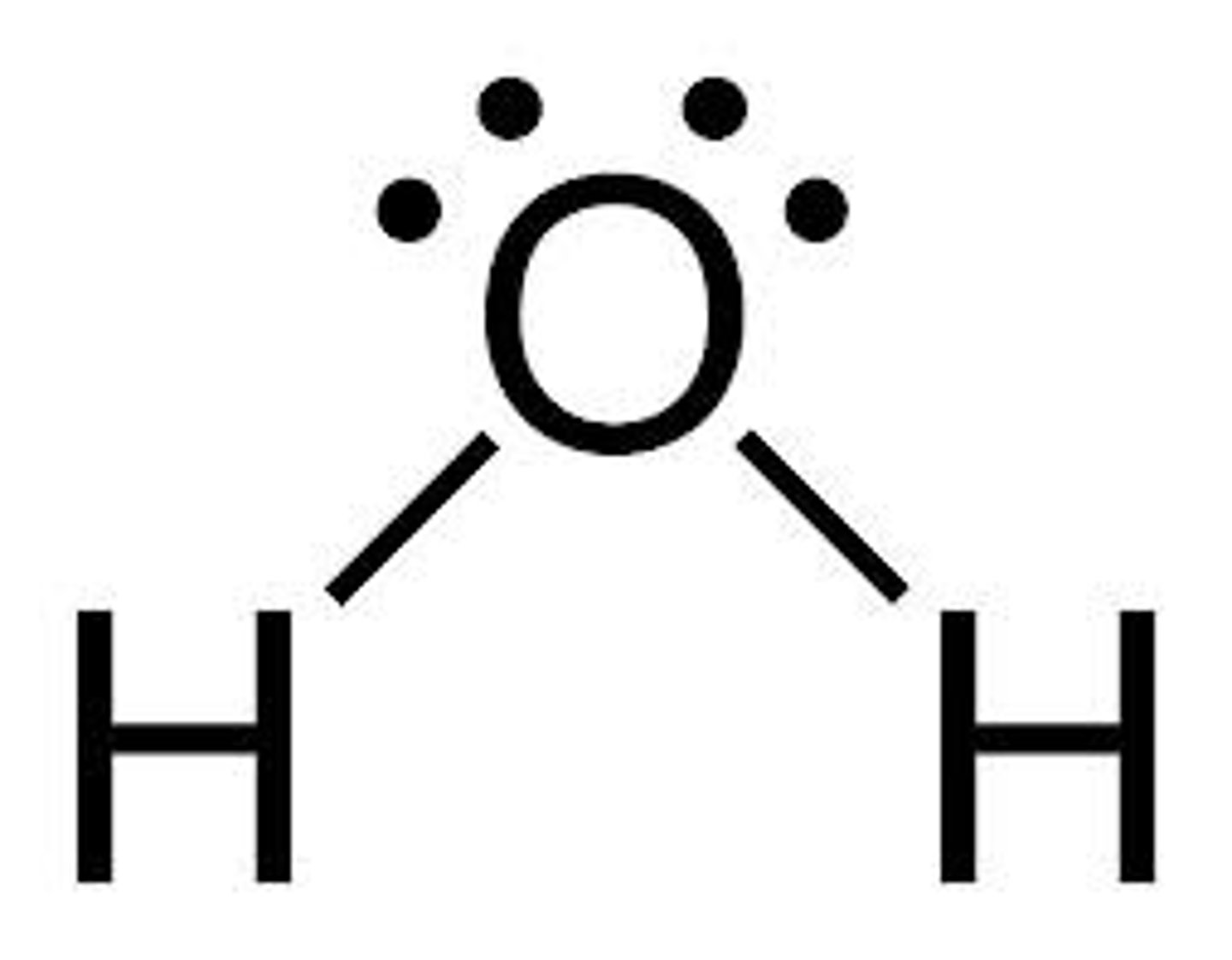
Transferred dot =
Ionic bond