Chemistry- Unit 1 Test
1/53
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Matter
Anything that takes up space and has mass
Pure Substance
Anything that has a fixed chemical composition and can’t be broken down physically
Element
A pure substance that is made of only one kind of atom
Compound
A substance that is made from the atoms of two or more types of atoms that are chemically bonded to one another
Heterogenous mixture
A mixture in which the components do not have a uniform composition
Extensive property
properties that change when the amount of the sample changes
Extensive examples
Mass, length, and volume
Physical property
A property that can be observed without changing the identity of the substance
Chemical property
A property that can only be observed by changing the identity of the substance
Solubility
The ability of a substance to dissolve in another
Burning, rusting, and reacting with another substance are examples of __________ changes.
chemical
Malleability, ductility, and flamability are all _______________
properties (descriptions)
Energy and mass must be conserved during a physical or chemical change, right gang?
conserved- maintain (a quantity such as energy or mass) at a constant overall total.
ya lol

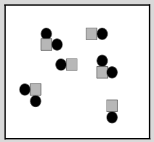
This image shows one type of atom. What best describes what this image represents?
an element

The ability for a substance to be hammered into a thin sheet is
malleability
Your ice melts in your water bottle. This is an example of a(n)
psysical change
A substance has a mass of 10 g and a volume of 2 mL. What is its density?
5g/ml
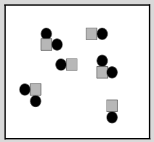
The image below shows two types of atoms bonded together. What best describes what this image represents?
mixture of compounds
Independent variable
What you change
A factor you change or control in an experiment, which you believe will influence another variable
Dependent variable
What you measure
A factor you measure or observe, which you think will change due to the independent variable
Property
The description of an object
If lightning strikes the tree will burn
The tree is 5 m high
The tree is GREEN)

Properties of matter can be
physical or chemical
Physical Properties (the def is simple and ez)
Are determined by the use of the five senses.
They are a description of an object.
(touch, hear, see, taste, smell)
Chemistry
the study of matter and the changes it undergoes
Ozone layer
located in the stratosphere (10-50 km above Earth)
made up of oxygen
a substance in the atmosphere that absorbs most harmful radiation before it reaches Earth’s surface.
Substance
also known as a chemical
matter that has a definite and uniform composition
a chemical is also known as
a substance
(matter that has a definite and uniform composition)
How is O2 formed?
Ultraviolet radiation from the Sun causes some oxygen gas ( O 2 )
Ultraviolet Radiation
If you have ever had a sunburn, you have experienced the damaging effects of ultraviolet radiation from the Sun.