chap 8a (atoms and ions)
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
What is matter?
Matter is a substance that has mass and takes up space i.e occupies a volume.
What are atoms?
Atoms are particles that matter is made up of.
What is an atom?
The atom is the smallest unit of an element that can take part in a chemical reaction.
Does an atom have an overall charge?
An atom has no charge.
What are the three sub-atomic particles in an atom?
Electron, proton and neutron.
Where are electrons found in an atom?
Electrons orbit around the nucleus in a path known as electron shell.
Why are electrons attracted to the nucleus?
The negatively charged electrons are attracted to the positively charged nucleus by electrostatic force.
What is the relative charge of a proton?
The relative charge of a proton is +1.
What is the relative charge of a neutron?
The relative charge of a neutron is 0.
What is the relative charge of an electron?
The relative charge of an electron is −1.
What is the relative mass of a proton?
The relative mass of a proton is 1.
What is the relative mass of a neutron?
The relative mass of a neutron is 1.
What is the relative mass of an electron?
The relative mass of an electron is 1/1836.
Why does the mass number not include electrons?
The mass number only includes protons and neutrons because electrons have negligible mass.
What determines the identity of an atom?
The identity of an atom depends on the number of protons.
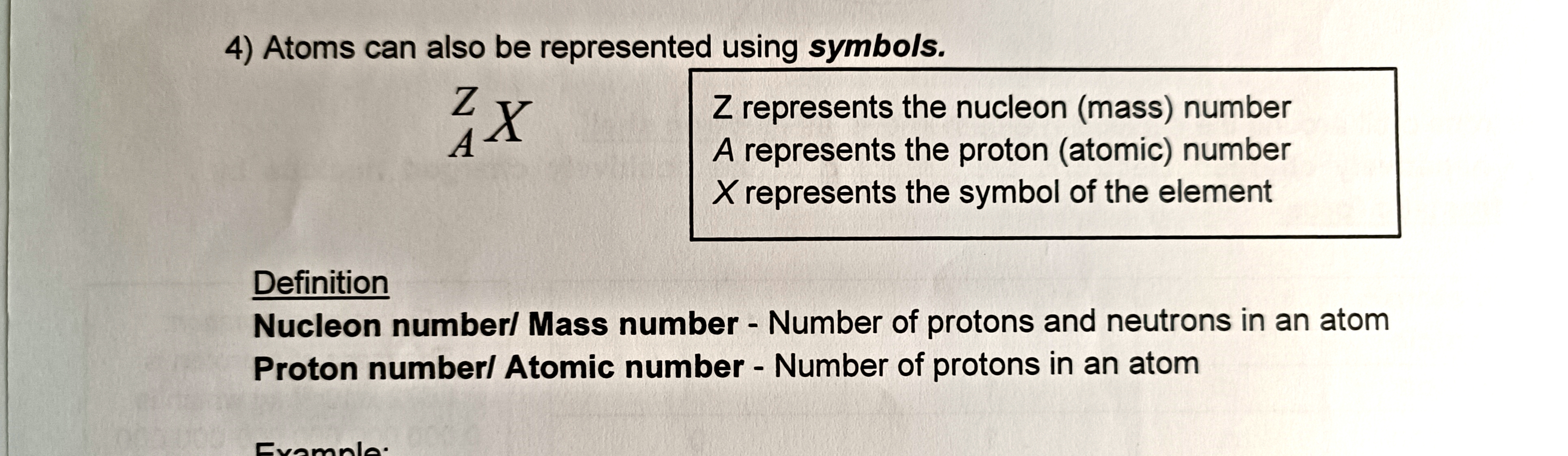
What is the nucleon number or mass number?
The nucleon number or mass number is the number of protons and neutrons in an atom.
What is the proton number or atomic number?
The proton number or atomic number is the number of protons in an atom.
What is the relationship between proton number, neutron number and mass number?
Proton number + neutron number = nucleon number (mass number).
How do you calculate the number of neutrons in an atom?
Number of neutrons = nucleon number − proton number.
How many electrons does a neutral atom have?
A neutral atom has the same number of electrons as protons.
Why is an atom electrically neutral?
An atom is electrically neutral because it has the same number of positively charged protons and negatively charged electrons.
What are the different ways atoms can be represented?
Atoms can be represented using diagrams showing sub-atomic particles, diagrams showing number of protons and neutrons, diagrams showing the identity of the element, and atomic symbols.

What are you expected to be able to do for representations of atoms?
You are expected to be able to recognize and draw representations of atoms using all three types of diagrams and make linkages to the atomic symbol. Diagram type 3 is the most common representation.
How is an atomic symbol written?
An atomic symbol is written with the symbol of the element, the mass number at the left hand top side and the proton number at the left hand bottom side.
What is an electron shell?
An electron shell is a region outside the nucleus where electrons can be found.
What is the maximum number of electrons in the first shell?
The first shell can only take 2 electrons.
What is the maximum number of electrons in the second shell?
The second shell can only have 8 electrons.
What is the maximum number of electrons in the third shell (for the first 20 elements)?
The third shell can only have 8 electrons.
What is electronic configuration?
The electronic configuration of an element shows how electrons in an atom are arranged in each electron shell starting from the innermost shell.
What is the valence shell?
The outermost electron shell is known as the valence shell.
What are valence electrons?
Electrons in the outermost shell are known as the valence electrons.
How do you find the group number for Group 1 and 2 elements?
For Group 1 and 2 elements, the group number corresponds with the number of valence electrons.
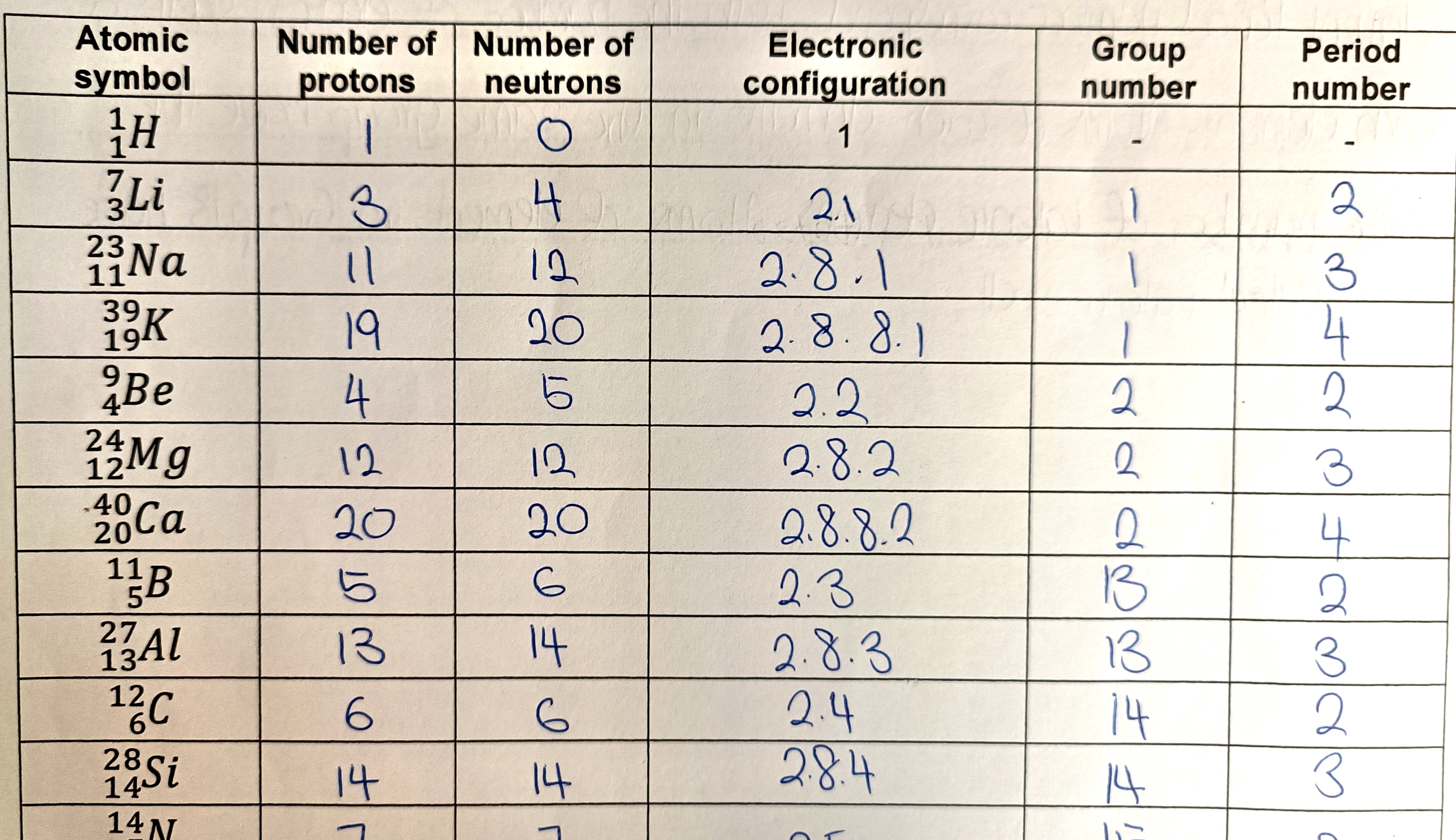
How do you find the group number for Group 13 to Group 18 elements?
For Group 13 onwards (specifically Groups 13–18), the group number corresponds with the number of valence electrons + 10.
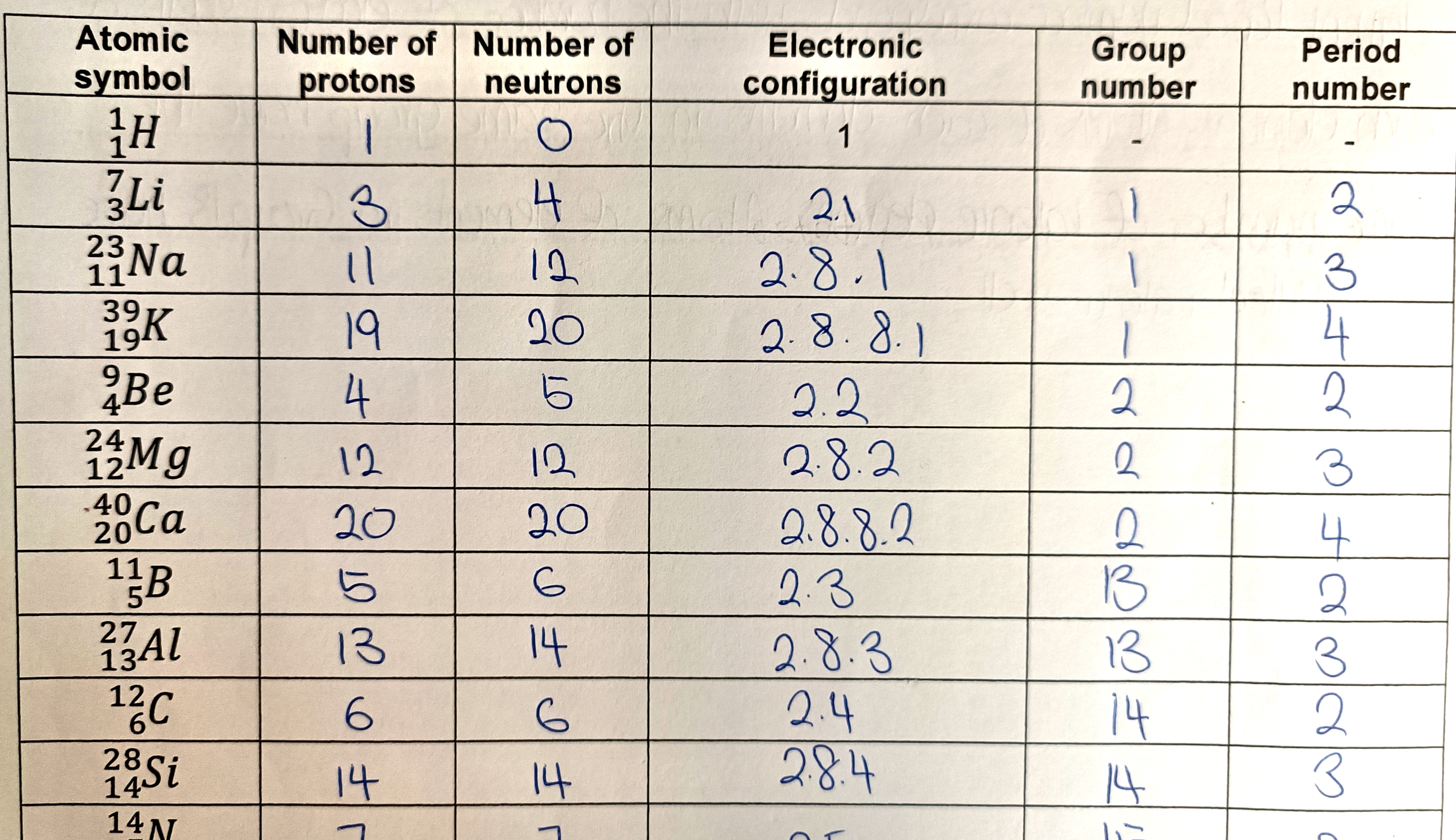
What do atoms in the same group have in common?
Atoms of elements in the same group have the same number of valence electrons.
How do you find the period number of an atom?
The number of electron shells an atom has is the same as the period number.
What is an ion?
An ion is an atom that carries a positive or negative electric charge as a result of having lost or gained one or more electrons.
Why do atoms form ions?
The primary reason atoms form ions is to achieve a full outer shell of electrons to attain stability.
What is a cation?
A cation is a positively charged ion.
What is an anion?
An anion is a negatively charged ion.
Where is the charge of an ion written?
The positive or negative sign is written on the right side of the chemical symbol and the drawing of the ion.
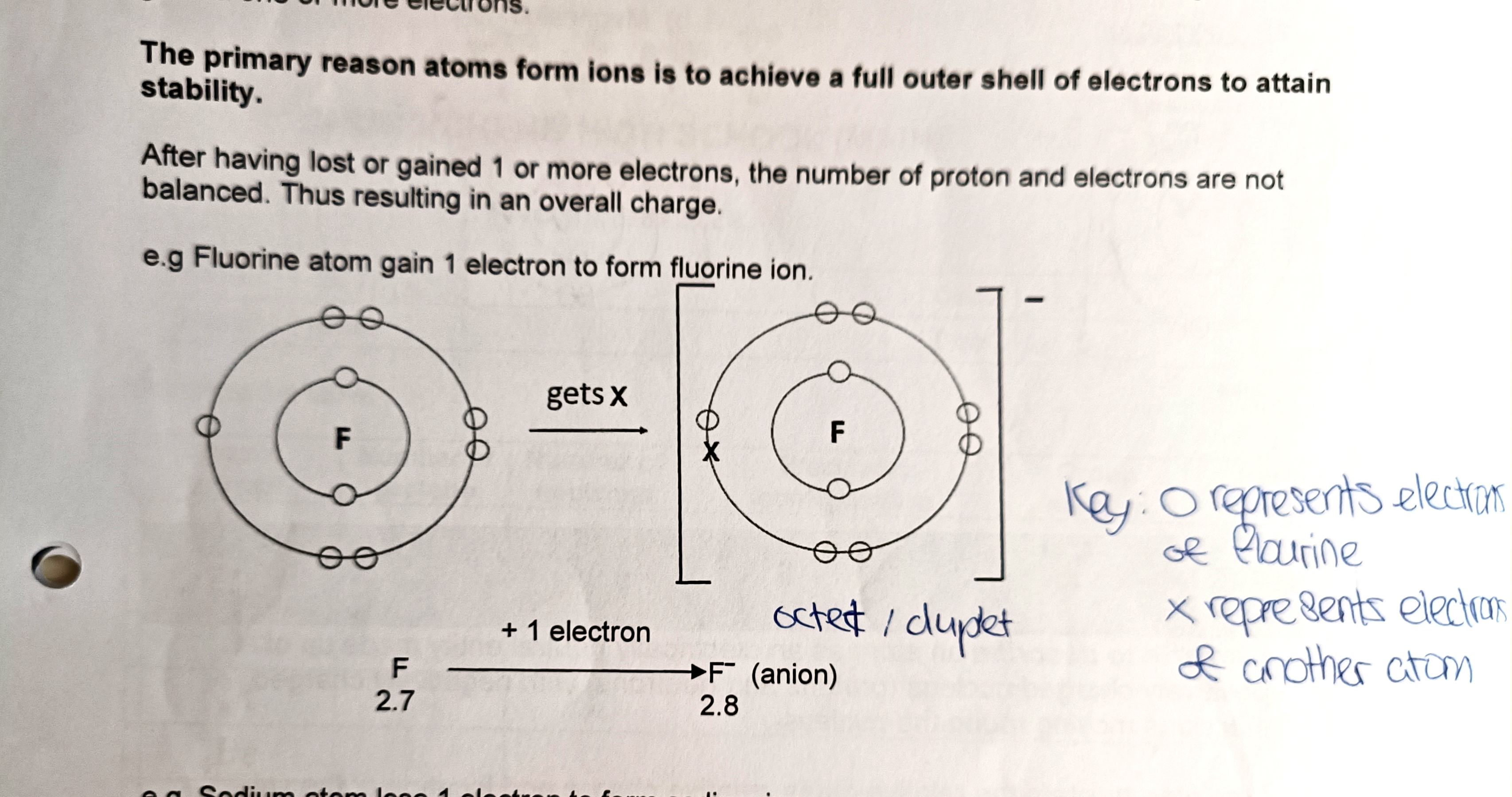
What are the steps to drawing an ion?
- Draw the atomic structure of the element and the added or deducted electron using a different symbol. 2. Bracket the drawing. 3. Indicate the charge of the ion outside the bracket at the top right hand side (for example: 2−, −, +, 3+).
What is an octet?
An octet is a full outer shell containing eight electrons.
What is a duplet?
A duplet is a full outer shell containing two electrons.
When drawing the atomic structure of an element, what must be included?
When drawing the atomic structure of an element, include a key of what each symbol represents (for example o represents an electron of another atom or element), an electronic configuration, its period number and group number depending on what the question asks for. Usually just the key is needed.