Graphene
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
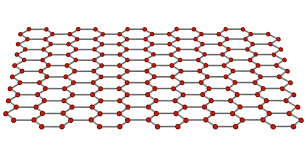
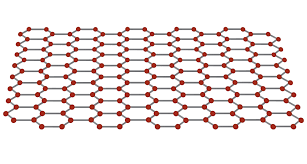
what structure does graphene have?
giant covalent structure.
2
New cards
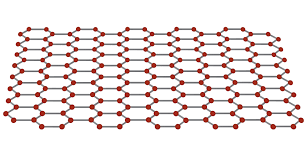
what structure is graphene arranged in?
hexagonal lattice as it is only 1 layer and one atom thick
3
New cards
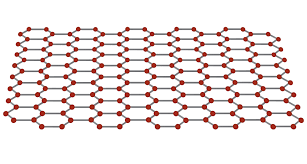
is graphene a conductor?
yes because it has delocalised electrons that are free to move around the structure.
4
New cards
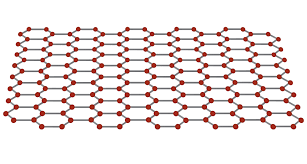
is graphene strong?
yes graphene is physically strong.
5
New cards
does graphene have a high melting point?
yes.
6
New cards
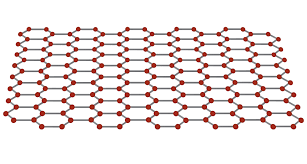
does graphene have strong bonds?
graphene has a strong covalent bond.
7
New cards
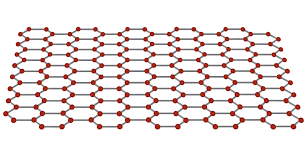
what is graphene useful for?
in electronics and for making composite.