Week 7: Arterial Blood Gases (and electrolyte balance)
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
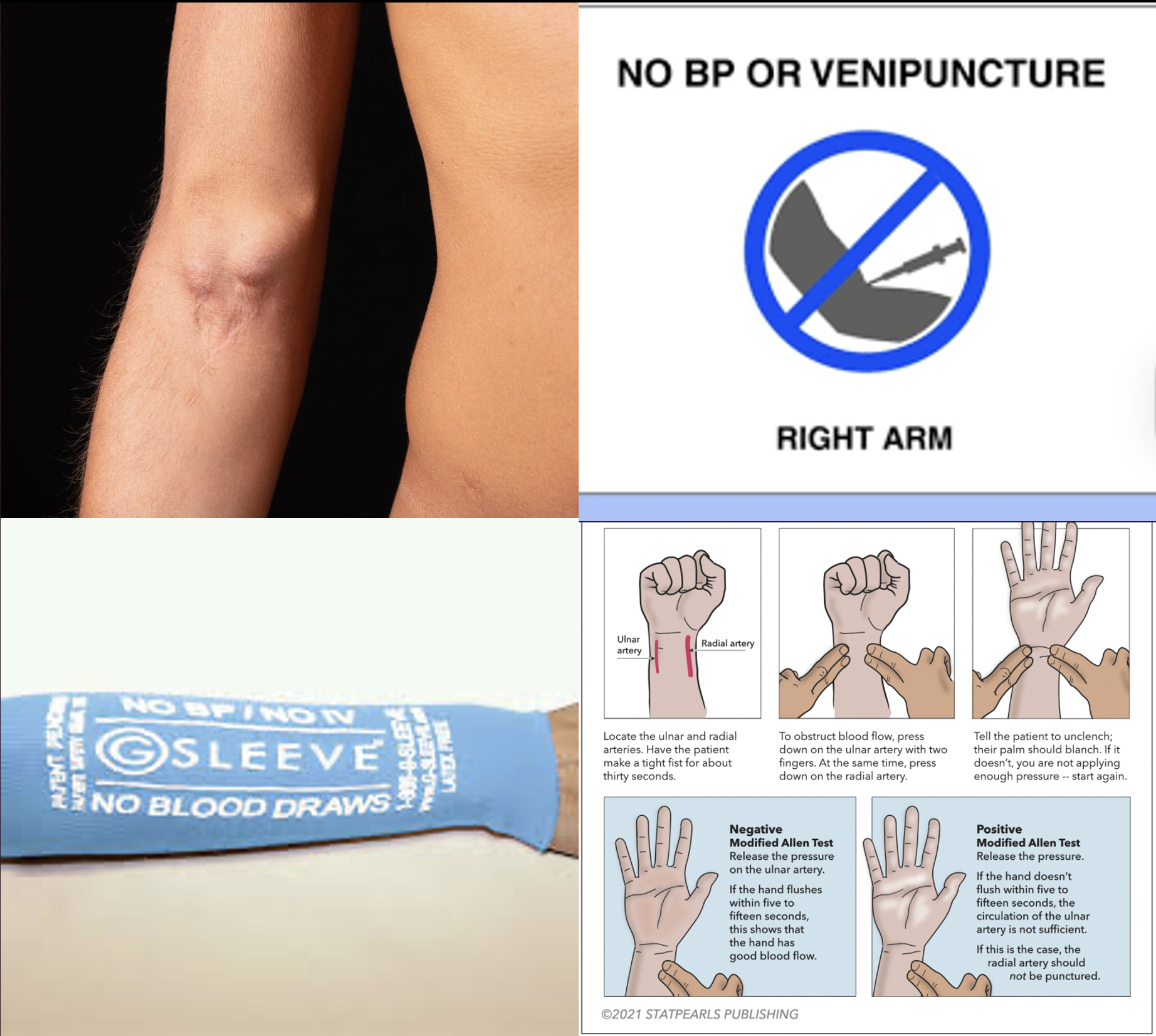
What are some considerations when selecting an IV and ABG draw site?
Contraindications to poking of the extremity: AV fistula, lymph node procedure, multiple pokes of the same site
Consider any hx of PAD or blood clotting disorders
Allen test if planning to use the radial artery (have pt make a tight fist to exsanguinate the hand, occlude both the radial and ulnar artery (and have pt release fist) until client experiences some numbness, paleness, or change in sensation; then release the ulnar artery and observe for return of color and sensation in the hand. Hand should flush within 5-15s if the pt has good blood flow. Lack of blood return indicates inadequate blood flow through the ulnar artery and that this radial artery should not be used.
Perfusion of the extremity: pulse, color, temperature, sensation
Also note before drawing ABG: Recent manipulation of the patient within the past 15 min that may influence their ABGs (e.g., tracheal suctioning, moving the patient, etc.)
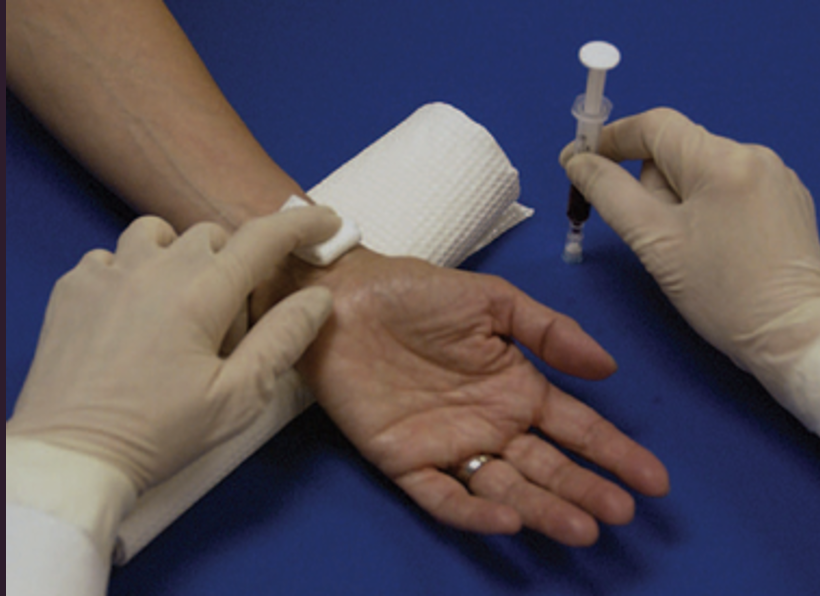
Nursing actions after drawing an ABG
Hold pressure for 5 min (10 min if on anticoagulants)
Verify patient identity as you label the specimen & transport it on ice immediately
Check for infection, hematoma, bleeding, thrombus (abnormal neurovascular status)
neurovascular status checks to assess for thrombi and ischemia
Learning to rapidly read arterial blood gases is imperative to detect problems in the body due to impaired gas exchange & ventilation, metabolic stress/impairment and cell death.
Being skilled in ABG interpretation can also help prevent downstream consequences of acid-base imbalance including impaired hormone, enzyme, and drug function, fluid and electrolyte imbalance, and altered membrane excitability of all organs.
In fact, alkalosis is generally associated with low electrolyte levels and acute acidosis is associated with elevated electrolytes.
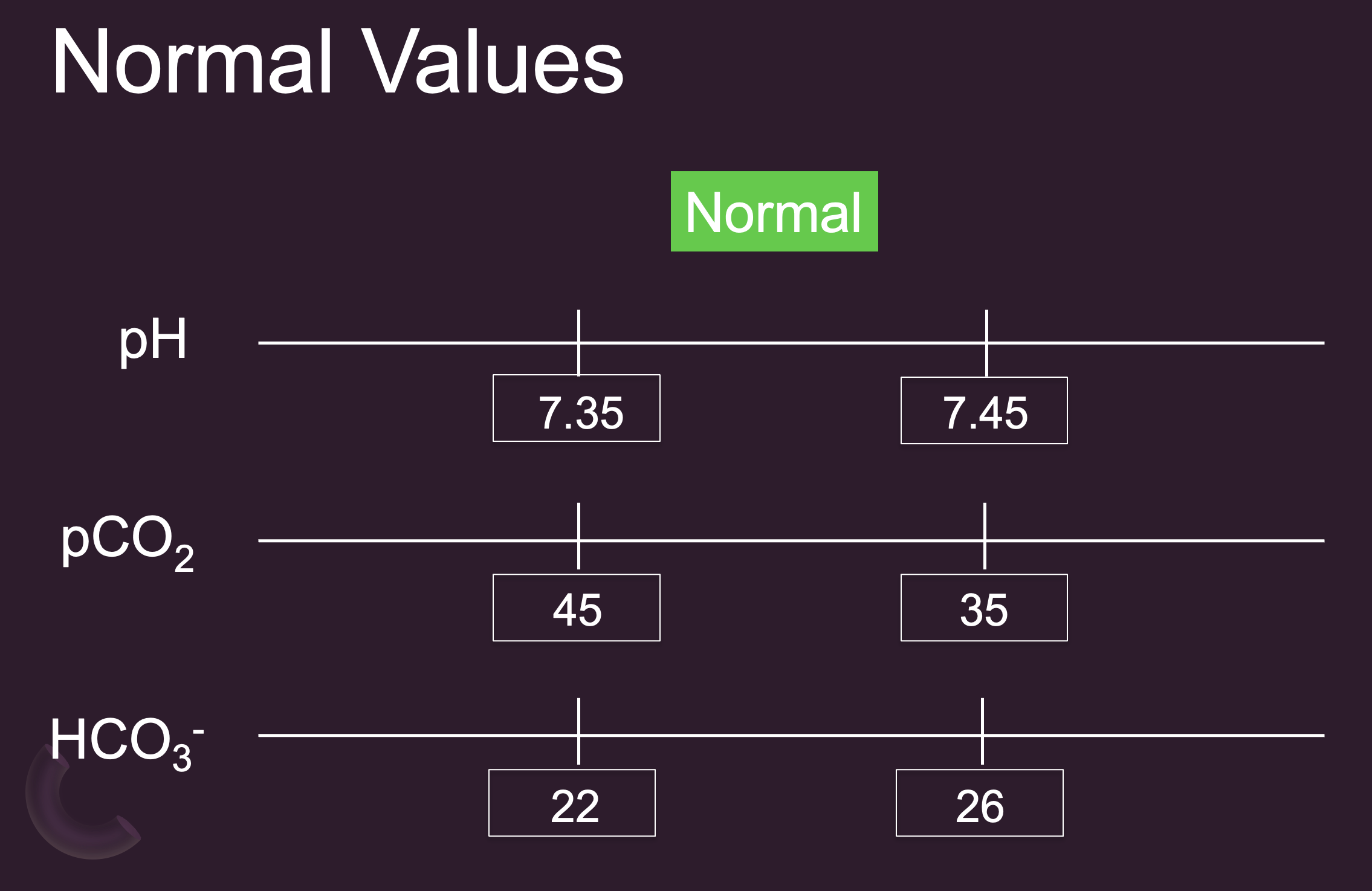
Normal Values
Normal Bicarb – some would say could extend to 21-28 mEq/L
Norm PaO2 = 80-100 mmHg
Acid-Base Control:
Acid-Base Control:
Buffer systems - Proteins (albumin, globulins). - Chemical (HCO3-, PO43-)
Respiratory system
Renal system
What happens:
When the rapid 1st responder chemical (ECF HCO3- & ICF PO43-) and protein (albumin, globulin, Hgb) buffer systems fail to compensate by binding to excess H+, the respiratory then renal systems kick in.
Respiratory drive in brain is controlled by H+ and CO2 levels (a very quickly responding mechanism).
Kidneys control HCO3-, PO43-, and ammonium (NH4+) creation & excretion, which drags H+ along. In acidotic states, the kidneys can create and reabsorb more bicarb into the blood to bind to excess H+. In this process of HCO3- creation, PO43- is formed in the urine and pulls H+ ions with it for excretion. Finally, the kidneys can use the excess H+ to convert ammonia (NH3) from protein breakdown to ammonium (NH4+) in the urine thereby increasing H+ excretion.
CO2 is an acid
Bicarbonate (HCO3) is basic
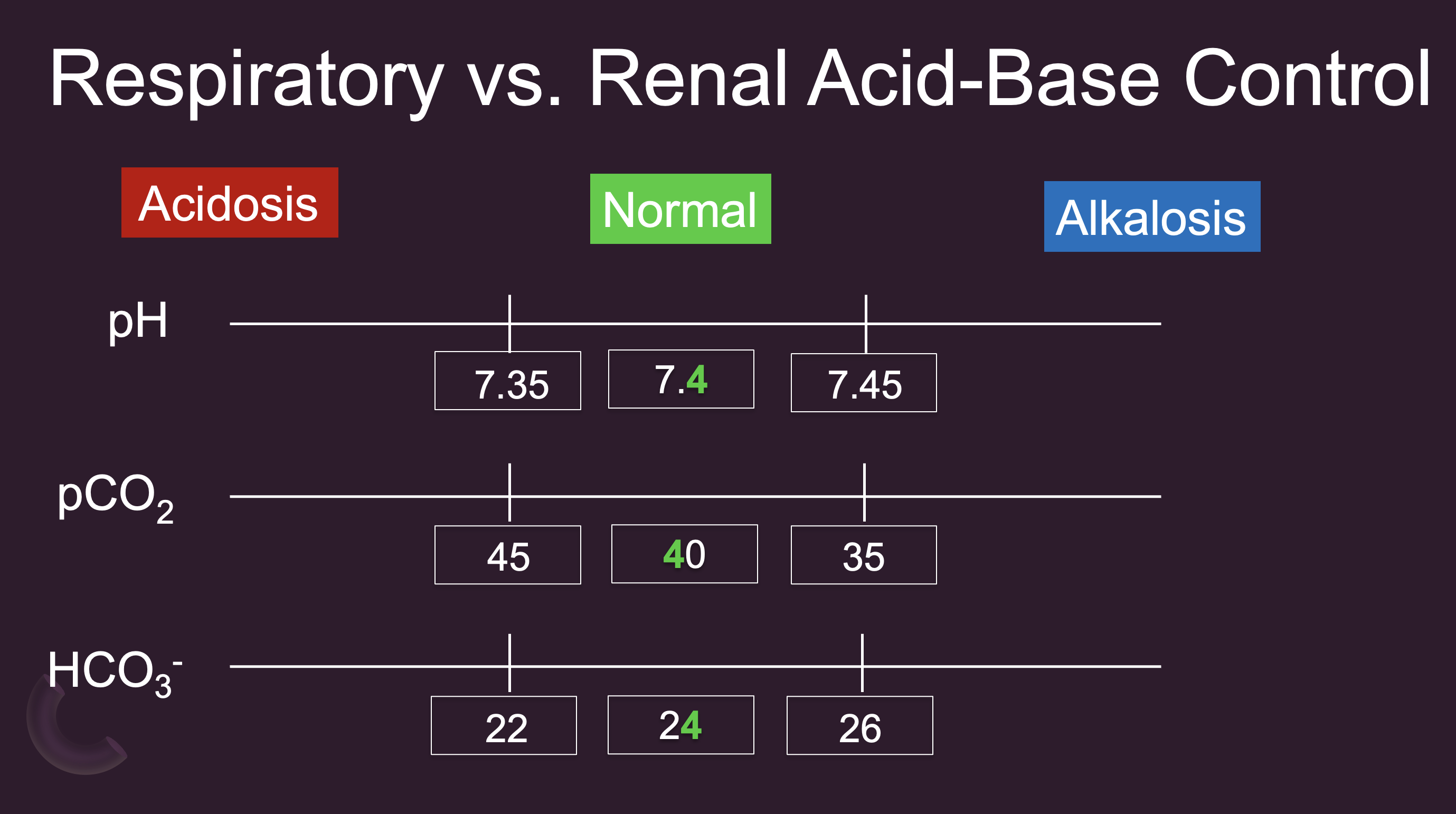
Respiratory vs. Renal Acid-Base Control
Given that CO2 is deemed acidic, higher levels of CO2 are aligned so they are on the acidic side.
Conversely, bicarb is basic, so higher bicarb levels is aligned with the alkalosis side.
Do you think the respiratory or metabolic system is most in control of CO2 levels? - Respiratory
Which system(s) would be most in control of bicarb? - Kidneys
How to read ABGs
Look at pH & CO2
pH → low is acidotic; high is alkalotic; normal could indicate compensation
pH & CO2 align (pH low and CO2 high) → respiratory; otherwise → metabolic
Look at HCO3-
If determine respiratory acidosis & HCO3- high or resp alkalosis & HCO3- low → compensation happening
Determine compensation or mixed depending on CO2 in & HCO3- direction
Determine if respiratory, metabolic, or mixed & whether compensated
Look at O2 to see if it is “with hypoxemia”
What are some causes of respiratory acidosis?
Causes: Respiratory depression & over sedation, inadequate lung expansion (e.g., pleural effusion, pneumothorax), airway obstruction/constriction (e.g., COPD, asthma), reduced alveolar-capillary diffusion (e.g., emphysema, PE, pulmonary edema, atelectasis, ARDS/decreased surfactant), improper use of mechanical ventilation, insufficient CPR, neuromuscular relaxants & disease.
What are symptoms of acidosis?
Terry Lynch, RN’s Rule: Anything bathed in acid is weaker, looser, slower
Anything bathed in alkaline is hyper, tenser, faster, irritable
Aligns with signs and symptoms of electrolyte imbalances
Electrolytes:
Hyperkalemia, magnesemia, calcemia, phosphatemia, chloremia, which (except for the hyperkalemia) → decreased excitable tissue
However, still matches up in terms of how the CV manifests with overall slowing of conduction.
CV – (all s/sx of hyperkalemia, hypermagnesemia, & hypercalcemia) Too much of these electrolytes interfere with the heart conduction, so you see slowing of conduction: Bradycardia/heart block, Peaked T waves, Widened QRS, Prolonged PR, Hypotension. Thready peripheral pulses
CNS: Depressed activity (lethargy, confusion, stupor, coma)
NM: Hyporeflexia, Skeletal muscle weakness, Flaccid paralysis
Resp: Kussmaul respirations indicate metabolic acidosis w/ resp compensation (rapid, deep, and labored breathing, fruity odor to the breath). Respiratory acidosis inadequate airway and breathing: bradypnea.
Skin: Warm, flushed, dry (in metabolic acidosis). Pale/cyanotic (in resp acidosis)
GI: N/V/D also associated with electrolyte imbalances
What management do you anticipate for acidosis?
Improve ventilation & oxygenation
Sit up; breathe deeper, harder, faster; O2 PRN
Sternal rub, narcan, bag valve mask, jaw thrust. (If constriction was present, consider bronchodilator like Albuterol, anti-inflammatories like fluticasone and singulair, and mucolytics.)
Attend to cardiac changes
Monitor ECG
Safety – Fall & seizure precautions
Fall and seizure precautions
Correct underlying condition & electrolytes
Continue monitoring
RR at least hrly, breath sounds, muscle retractions & use of accessory muscles, expansion, nail beds & oral membranes for cyanosis
What are causes of respiratory alkalosis?
Excessive loss of CO2: hyperventilation: fear/anxiety, Mechanical ventilation, Salicylate toxicity, High altitudes, Early-stage acute pulmonary problems
What are symptoms of alkalosis?
Terry Lynch, RN’s Rule: Anything bathed in alkaline is hyper, tenser, faster, irritable
Aligns with signs and symptoms of electrolyte imbalances
Electrolytes:
Hypokalemia, magnesemia, calcemia, phosphatemia, chloremia → increased excitable tissue (due to the low electrolytes, except for potassium)
CNS: Increased activity, Anxiety, irritability, tetany, seizures, Chvostek sign, Trousseau sign, Paresthesias
NM: Hyperreflexia, Muscles cramping/twitching, Skeletal muscle weakness
CV: Increased or variable HR but weak thready contraction & pulses (might see inverted T waves/depressed ST segment). Normal/low BP, Increased dig toxicity
Resp: Hyperventilation if due to resp alkalosis. Decreased resp effort d/t skeletal muscle weakness in metabolic alkalosis. Metabolic – breathing slower, decreased respiratory effort, may be compensatory
What management do you anticipate?
Fall precautions
Abdominal/diaphragmatic and/or pursed lip breathing
Provide calm reassurance
Supplemental O2 PRN
Continue monitoring bicarb
What are cause of metabolic acidosis?
Causes of Metabolic Acidosis:
Overproduction of hydrogen ions (DKA, starvation, sepsis, lactic acidosis-anaerobic respiration)
Underelimination of hydrogen ions (severe lung impairment w/ CO2 retention, kidney failure & uremia)
Underproduction of HCO3 (kidney failure, impaired liver or panc function)
Overelimination of HCO3 (diarrhea, ureteroenterostomy, pancreatic fistula)
Toxins: Alcohol, methanol, paraldehyde, Iron OD, Ethylene glycol, salicylate, acetazolamide
What management do you anticipate? (metabolic acidosis)
Hydration
Attend to cardiac changes
Safety – Fall & seizure precautions
IV Insulin
IV Bicarbonate if pH<7.2
Management for DKA: Assess for cardiac changes and give fluids and insulin to correct hyperglycemia, hyperkalemia, and prevent further ketone production. Maintain pt safety.
Sodium bicarbonate if serum bicarb low and pH<7.2
If GI-induced metabolic acidosis, administer antidiarrheals or antiemetics and/or get rid of the causative agent.
What are cause of metabolic alkalosis?
Increased base
Oral ingestion of bases (antacids)
Blood transfusions
HCO3 infusion
TPN
Decreased acid
Prolonged vomiting
HG suctioning
Hypercortisolism
Hyperaldosteronism
High loop and thiazide diuretic doses
What management would you anticipate? (metabolic alkalosis)
Management:
NS infusion
Correct low electrolyte levels
Possibly HCl infusion (rare)
Stop inciting medications & correct underlying cause
Mixed Respiratory and Metabolic Acidosis
Occurs in patients with hypercapnic acute respiratory failure, in this case, during COPD exacerbation (air trapping), concurrent with metabolic conditions such as CKD exacerbation.
Other metabolic conditions that could occur concurrently with respiratory acidosis include rhabdomyolysis, DKA, or metformin-related lactic acidosis
Initial Management: Nebulized bronchodilators, intravenous corticosteroids, antibiotics (if necessary) and other drugs, depending on the concomitant comorbidities
CPAP/BIPAP if previous interventions ineffective
Which 3 compounds are formed in the kidneys to buffer and/or assist in excreting H+?
Bicarbonate (HCO3-), Phosphate (PO43-), Ammonium (NH4+)
What integumentary assessment findings are associated with metabolic acidosis?
Warm, flushed, dry skin (compared to pale or cyanotic dry skin in respiratory acidosis)
What is the earliest sign of acid-base imbalance?
Altered mental status
What is the most common condition associated with bicarbonate hyperexcretion?
Diarrhea
A patient on nasogastric suctioning is at risk for which type of acid-base imbalance?
Metabolic alkalosis
Seizures and other hypermetabolic states causes anaerobic glucose breakdown and what specific type of acidosis?
Metabolic lactic acidosis
What are 3 OTC drugs/substances associated with acid-base imbalances? Explain the type of acid-base imbalance each medication is associated with.
Antacid - metabolic alkalosis;
Aspirin - metabolic acidosis;
Alcohol - mixed metabolic and respiratory acidosis
What is the most important assessment to perform after drawing an ABG sample?
Neurovascular assessment (extremity sensation, color, temperature, movement, pulses)
What is the most common first action to manage metabolic acidosis?
Hydration/Fluid resuscitation
What assessment should be performed when choosing whether to use the radial artery to draw an ABG sample?
Allen Test - (occlude both the radial & ulnar artery until client experiences some numbness, paleness, or change in sensation; then release the ulnar artery and observe for return of color and sensation in the hand)
What first 3 actions should be implemented to manage respiratory acidosis due to COPD exacerbation?
Sit patient up; administer drugs to open the airway (bronchodilators, anti-inflammatories, and mucolytics); administer oxygen
Given the neuromuscular effects of acid-base imbalances, what is the nurse’s top priority in maintaining patient safety?
Implementing fall precautions