organic analysis II
1/141
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
142 Terms
describe the process of thin-layer chromatography (TLC)!
Take a sheet/plate of glass, plastic or metal.
Coat it with a thin layer of silica gel or alumina.
Draw a pencil line near to the bottom.
Place a small drop of the sample on this line.
Place the plate in a beaker containing solvent, but not enough to reach the pencil line.
Cover the beaker.
(As the sample rises up that plate it will separate into its components)
Thin layer chromatography vs paper chromatography
Thin layer chromatography uses a thin layer of silica (SiO2) or aluminium (Al2O3) spread on a sheet of glass or metal. Paper chromatography uses paper instead. This means the components spread out more which makes it harder to analyse
What is the moving phase
moving phase is whatever moves the components. E.g in thin layer chromatography the MP is always a solvent
What is the stationary phase
stationary phase is whatever the moving phase moves through E.g in thin layer chromatography the SP is always alumina or silica gel
The distance a component moves can be partly explained by 2 factors:
FACTOR 1 : the level of retention of the component by the stationary phase. E.g Retention will be high if both the component and the stationary phases is polar or could form hydrogen bonds.
FACTOR 2 the level of solubility of the component in the moving phase. The level of solubility is high if for example both the solvent and component are polar or if hydrogen bonds can form between them
How do the interactions between the stationary phase and different components affect their retention in thin-layer chromatography (TLC) using silica or alumina as the stationary phase?
Silica (SiO₂) and alumina (Al₂O₃) are polar stationary phases, so polar components like 1-chloropropane ADSORB strongly, resulting in high retention and minimal movement up the stationary phase.
Non-polar compounds (e.g., cyclohexane): Weaker interactions with the stationary phase → Low retention → Move further up the plate.
In thin-layer chromatography (TLC), using silica gel as the stationary phase and pentane as the mobile phase, explain why 1-chloropropane moves less distance up the plate compared to cyclohexane.
1-chloropropane is polar so its not as soluble in pentane (non polar). It has greater retention by the silica gel (polar) so a molecule of 1-chloropropane spend most of its time adorbs to the stationary phase and only some fo it time in the moving phase so it doesn’t move very far up.
In thin-layer chromatography (TLC), using silica gel as the stationary phase and pentane as the mobile phase, explain why 1-chloropropane moves less distance up the plate compared to cyclohexane.
1-Chloropropane (polar):
Low solubility in pentane (non-polar) → spends more time adsorbed to the polar stationary phase (silica gel).
High retention → Moves less distance up the plate.
Cyclohexane (non-polar):
High solubility in pentane (non-polar) → spends more time in the moving phase (pentane).
Low retention → Moves further up the plate.
Summary of retention
High retention = more time in stationary phase + less movement up the paper. Retention will be high if both the component and the stationary phases is polar or could form hydrogen bonds.
Summary of solubility
HIgh solubility = less time in stationary phase + more movement up the paper. The level of solubility is high if for example both the solvent and component are polar/both are non polar (like dissolves like) or if hydrogen bonds can form between them
factors affecting movements up the plate
What is the Rf equation
To find a component Rf value:
Wait until the solvent has moved up the plate and then take it out and mark in pencil how far the solvent travelled
Label the start line
Label the solvent front (which is the distance travlled by solvent from the start line ) and measure the length of the line
Then draw another line and measure the distance traveled by the component from the start line
Calculate rf (2dp) = distance traveled by component/distance traveled by solvent. - C is before S in alphabet - remember Rf value is always to 2dp
What is the term for the result of a chromatography experiment?
When we are analysing the results of chromatography we call the thing we are analysing a chromatogram
How do we calculate Rf values in thin-layer chromatography and how are they reported?
We record the results of thin layer chromatography as Rf values.
Rf value = Distance travelled by the component / Distance travelled by the solvent
Reported to 2 decimal places.
What does an Rf value tell us in thin-layer chromatography?
An Rf value tells us how far a component travels as a fraction of how far the solvent travels.
Rf close to 1: Component is near the solvent front (moves far).
Rf close to 0: Component is near the start line (moves little).
What is this components Rf
This components Rf value is around 0.80 (2dp) because it is around 80% of the way from the start line to the solvent front
A common mistake is people mislabeling the solvent front so remember to label this at the further distance traveled by the solvent and not just the end of the paper (a common mistake).
Also don’t forget it is 2dp.
Name two methods which can be used to see components on chromatogram which don’t show up under normal circumstances.
Spray a developing agent on a chromatogram to make the dots visible OR
Add a substance which fluoresces under UV light to the stationary phase. (so that when once we’ve run the chromatogram you shine a UV light and everything glows except from one of the components)
What is the advantage of column chromatography?
Column chromatography makes it possible to collect the components after they have been separated
What are the steps involved in column chromatography?
We need a stationary phase like Silica (SiO₂), alumina (Al₂O₃) or resin and a solvent for the moving phase
Pack the stationary phase into a column/narrow glass tube
Add the sample to the column.
Add the solvent (eluent) to wash down and separate the components.
Explain how the choice of stationary phase and moving phase affects the separation of components in column chromatography.
The moving phase washes the sample through the stationary phase, separating components based on their solubility in the moving phase and retention by the stationary phase. A highly polar component will travel faster if the moving phase is polar (high solubility) and the stationary phase is non-polar (low retention).
What is the moving phase called in column chromatography, and what term is used to describe a component leaving the column?
n column chromatography we call the moving phase the eluent instead of solvent.
When a component is trickling down the bottom of the column we say that it is eluting
Outline gas chromatography
Gas chromatography separates components like column chromatography and identifies them like thin layer chromatography.
The sample moves through a coiled tube heated in an oven, with temperature control.
The stationary phase is a powder or a powder coated in a viscous liquid (like oil).
An unreactive gas (helium or nitrogen) is the moving phase.
Since the moving phase is a gas, it can't dissolve the components, so we only consider their affinity for the stationary phase.
Nonpolar components pass through fastest if the stationary phase is polar.
A detector at the end produces a gas chromatogram graph.
The components on a gas chromatogram is shown on a graph like this:
How do we determine the abundance of a component in gas chromatography?
Height & Width of Peaks: The height of each peak shows how much of the sample eluted, and the width of the peak shows how long the sample was eluting.
Area Under the Peak: The area under the peak represents the component's abundance.
Key to Abundance: The larger the area, the greater the abundance of that component in the sample.
While C is the highest peak A is most abundant because of both its height AND width meaning it has a larger area underneath the peak
While C is the highest peak A is most abundant because of both its height AND width meaning it has a larger area underneath the peak
What is retention time
In column chromatography and gas chromatography, we measure retention time.
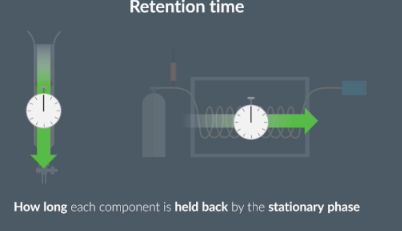
What is retention time affected by
Retention time can be affected by retention by the stationary phase and solubility in the moving phase.
For example, if a polar stationary phase is used in gas chromatography, the component with the higher retention time will be the polar component
ethene
chloromethane
Ethene is non polar so has a high retention time. Chloromethane is polar so has a low retention time.
How to find the retention time in a gas chromatogram
To find retention time in gas chromatogram we draw a line down form the top of the peak and read it off against the x axis.
: How can gas chromatography identify components on its own?
On its own, gas chromatography tells us each component’s retention time which can be enough to identify it . SO id we use exactly the same conditions just knowing the component retention time might be enough to identify it - chemists have a lost of compounds with their retention times
What is GC-MS and how does it enhance gas chromatography?
GCMS is when we replace the detector in gas chromatography with a mass specotomertes. It can tells us each component’s retention time, mass and fragmentation pattern (if we use electron impact as our ionization method)
This combination helps in identifying components more accurately by giving both physical (retention time) and chemical (mass and fragmentation) data.
how to idneitfy cabron environments

Identifying carbon environments
Once we have drawn 3 tables for the 3 carbons in the molecule and noted the number of atoms that are bonded to 1,2, etc bonds away we can compare the tables to identify carbon environments
These 2 tables highlighted are the same so they are in the same carbon environments
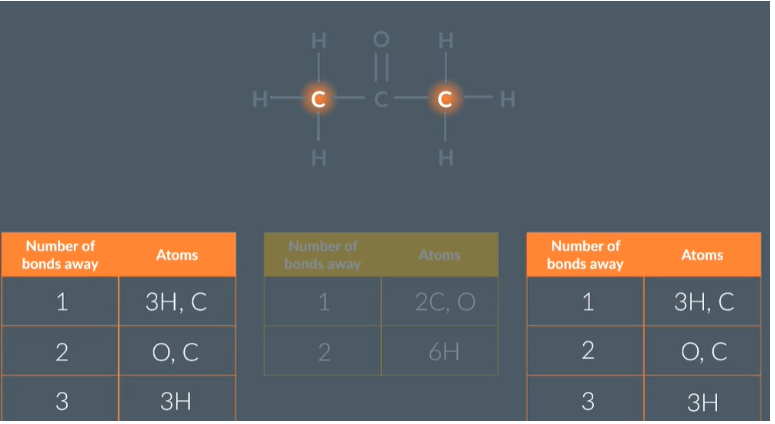
(NMR tells us the number of carbon environments in a molecule)
How does NMR spectroscopy determine different carbon environments in a molecule?
NMR spectroscopy identifies how many different carbon environments are in a molecule.
Two carbons are in the same environment if:
They have the same set of neighbouring atoms.
They are the same number of bonds away from those neighbouring atoms.
Symmetry Shortcut 1
Whenever we can reflect a molecule so that it looks EXACTLY THE SAME as it did before we reflected it, any two carbons which swap places as a result of that reflection must be in the same environment.
Symmetry Shortcut 1
Once we have worked out which carbon are in the same environment. We then take one carbon from each same environment pair alongside any carbons which are symmetry shortcut didnt help us with and use the table method to compare them
Symmetry Shortcut 1
There is no way of flipping this molecule so it looks the same originally. This shortcut doesn't work for every molecule. Some molecules when flipped won't look the same as it did originally therefore we cant use this method (we use the table method instead)
Symmetry Shortcut 2
Whenever we can rotate a molecule so that it looks exactly the same as it did before we rotated it, any two carbons which swap places as a result of that rotation must be in the same environment.
Symmetry Shortcut 2
Once we have worked out which carbon are in the same environment. We then take one carbon from each same environment pair alongside any carbons which are symmetry shortcut didnt help us with and use the table method to compare them
Symmetry Shortcut 2
There is no way of rotating this molecule so it looks the same originally (unless we rotate it 360 but this doesn't change the position of any carbons). This shortcut doesn't work for every molecule. Some molecules when rotated won't look the same as it did originally therefore we cant use this method (we use the table method instead)
Symmetry shortcut 3
Whenever we can rotate a bond such that the molecule looks exactly the same as it did before the rotation, any two carbons that swap places must be in the same environment. We can only rotate single bonds NEVER double bonds
Symmetry shortcut 3
Once we have worked out which carbon are in the same environment. We then take one carbon from each same environment pair alongside any carbons which are symmetry shortcut didnt help us with and use the table method to compare them
Symmetry shortcut 3
There is no way of rotating bands in this molecule so it looks the same originally (unless we rotate the bond 360 but this doesn't change the position of any carbons). This shortcut doesn't work for every molecule. Some molecules bonds when rotated won't look the same as it did originally therefore we cant use this method (we use the table method instead)
highly symmetrical molecules
Some moeclues are high symmeritrical and for those we can use multiple symmertriy shortcuts at once
disguised similar environments
Sometime the way a skeletal molecule is drawn can disguise the symmetry in a molecule but if you can redraw the molecule in a way that you can use a symmetry shortcut they still work,
disguised similar environments
There is no way of redrawing it in a way that shows a symmetry shortcut so all its carbons are in different environments but use the table method to double check
counting carbon environments
Counting carbon environments is a skill you will need to take every NMR question and this is the steps you use.
Step 1 - use symmetry shortcuts to identify carbons in the same environment
If we rotate the yellow bond these two carbons are in the same environment
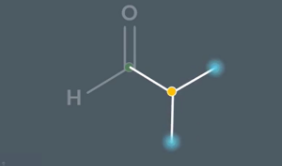
Once we have worked out which carbon are in the same environment. We then take one carbon from each same environment pair alongside any carbons which are symmetry shortcut didnt help us with and use the table method to compare them
E.g
Most of the time these selected carbon atoms will all be in different environments but it's worth double checking using the table method (Step 3)
counting carbon environments - step 3
E.g counting carbon environments from skeletal formula
In this case we only have to look at carbons one bond away to know that there are in different carbon environments.
Yeollow is 3 carbons and 1 hydorgen bceause it has 3 cabron bonds and each carbon nneds to have 4 bonds. Sketelal formuals dont highlight hydrogen bonding so it must be bonded to 3 cabron and 1 hydrogen
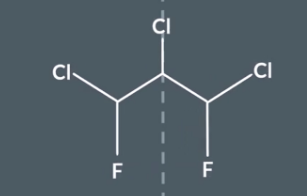
This is an example of disguised symmetry - IF we rotate one of the C-F-CL and then reflect it there is symmetry but we might not notice this. But we can still get out correst answer form using the method.
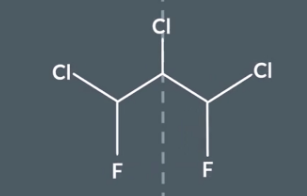
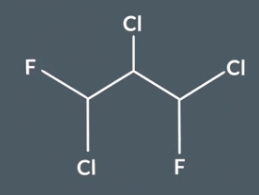
Step 1 - use symmetry shortcuts to identify carbons in the same environment
In step 1 we would have found nothing
Step 2
\In step 2 we would have selected all 3 carbons
Step 3
Using the table method.
Using the table method.
Using the table method.
If we look 1 bond away we can identify that 2 of 3 carbons are in the same environment and 1 of the carbons is in a different environment.
IF we look 2/3/4 etc bond away we can see that the 2 carbons are the same carbon environment. This means that the molecule has 2 carbon environments
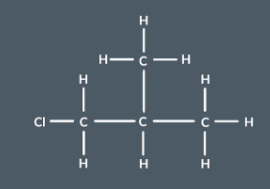
What do we do if we are asked to find the number of carbon environments using a molecules displayed or structural formula
I
f we are asked to find the number of carbon environments from a molecules displayed or structural formula we first convert to skeletal formula and continue with the procedure as usual.
For example
For this molecule if we use the table method we have to chart all the atoms 1-4 bonds away before finding out they are in different environments
shortcut to the table method: a unique molecule
However if we focus on a unique molecule (F atom) and count the number of bonds it is away from each carbon we can identify if they are in the same environment.
For example F is 4 bonds away from the orange carbon and 5 bonds away form the green carbon. SO these 2 carbons are in different environments
shortcut to the table method: a unique molecule
Wherever there is a unique atom in a molecule that means if 2 carbon atoms are a different number of bonds away from it then they must be in different environments
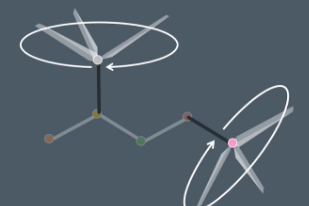
visualising symmetry in 3D
When counting environments whenever a molecule does not have any bonds to hydrogen atoms its useful to visualize the molecule in 3D and a helpful way to do that is by imaging 3 of the bonds carbon makes as helicopter blades and the 4th bonds as the pole they are rotating around
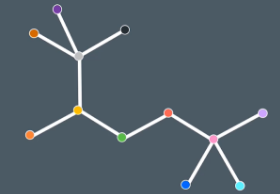
Take a look at this molecule which of the carbons are in the same environment
The top 3 carbons are all in the same environment and the bottom 3 bonds are in the same environment
What does the number of peaks in an NMR spectrum tell us?
If we run NMR on a sample of a molecule, the number of peaks in the NMR spectrum produced is equal to the number of carbon environments in the molecule.
What does the position of peaks in an NMR spectrum tell us?
The position of peaks in an NMR spectrum provides information about what atoms are bonded to the carbons in each environment, such as:
220-150 → Carbons double-bonded to oxygen (C=O)
100-50 → Carbons bonded to oxygens (C-O)
50-0 → Carbons bonded to carbons and hydrogens (C-C, C-H)
Explain why ^12C nuclei are not detectable by NMR spectroscopy, whereas ^13C nuclei are
Some nuclei have spin and if they do they can be analysed by NMR. E.g the nucleus of a C-12 atom does not have spin so it can’t be analysed by NMR but the nucleus of a C-13 atom does have spin so it can.
describe how an external magnetic field influences the spin states of ^13C nuclei
. All C-13 nuclei are in 1 of 2 possible spin states. Usually the energy of both spin states are the same but when they are in magnet their energies become different and the energy gap between them is what NMR takes advantage of (stronger the magnets the larger the energy gap)
How does the environment a carbon 13 is in affect the size of the energy gap?
When we put a molecule near a magnet the spin states of each of it carbon 13 nuclei split in energy and the size of the resulting energy gap is affected by their neghbouring atoms. If 2 carbon 13 atoms are in the same environment their energy gaps will be the same but if they are in different environments their energy gaps will be different
If C-12 5+ was an option we can ignore it because C12 has no spin
How does the electron density around a C-13 nucleus have an impact on the energy gap between its 2 spin states
When a carbon-13 nucleus is placed in a magnet, an energy gap arises between its two spin states.
The lower the electron density around the nucleus, the larger the energy gap.
How does fluorine affect the energy gap in Carbon-13 NMR?
The carbon 13 next to fluorine has a larger energy gap than the carbon next to hydrogen. This is because the cabron are in different environment. Fluorine is very electronegative so it removes electron density form the carbon.making its energy gap larger
These energy gaps are closer in size but still different because the cabrons are in different environments. Specifically 1 of the C-13 is close to the F so it has less electron density around it.
These energy gaps are closer in size but still different because the cabrons are in different environments. Specifically 1 of the C-13 is close to the F so it has less electron density around it.
If we swapped the fluorine for a hydrogen the energy gaps are even closer together but still different because the carbon are in different environments.even without the flurien they are still not surrounded by the exact same number of atoms
When do Carbon-13 nuclei have identical energy gaps?
To have identical energy gaps between spin states, the carbon-13 nuclei in a molecule must have the identical electron densities around them.
And that’s only possible if the carbons are in the same environment.
Explain how carbon-13 (^13C) environments are analyzed using NMR spectroscopy.
Whenever molecules are placed in a magnet, each carbon-13 environment will have a certain frequency of radio wave which causes its nuclei to switch spin states
How to investigate the different C-13 environments in a sample using a NMR spectrometer .
To investigate the different C-13 environments in a sample we just take a sample of that substance, put it in a magnet, shine radio waves on it and detect what frequencies of radio waves it interacts with
If we look at this table the last ethanol molecule is the only atom with only C-13 and it is very very rare BUT NMR can detect this
But if we put all of these molecules of ethanol in a sample The NMR will detect the frequencies of C-13 atoms
Why don't we plot NMR data on standard graphs?
We don’t plot NMR data on graphs like the one shown above, and that’s because different NMR spectrometers would produce different graphs for the same sample (due to differences in the strength of the magent)
To ensure graph are standardized and look the same:
We add a standard to the sample before we run each NMR. We tell the computer linked to the spectrometer to plot frequencies relative to that standard and the result is a spectrum that looks like this
What is the x-axis of an NMR spectrum measured in, and what does a chemical shift represent?
We call the values on the x axis shifts and they are measured in parts per million. Each spectra shows shifts between 0 and 220 ppm with 0 on the right hand side. If a peak has a shift of 23 ppm that means it corresponds to a frequency that is 23 millionths larger than the frequency of the standard peak
The standard we use in NMR is called
Tetramethylsilane
5 reasons why we use tetramethylsilane as our standard
Sometimes in an exam question you will be shown a selection of molecules and a NMR spectrum. You’ll be asked to identify which molecules produces the NMR spectrum. To do this
Identify the number of carbon environments in each of the molecules
IF the number of cabron environments does not match the number of peaks on the spectrum then that option must be incorrect
Sometimes a question will give you the molecular formula of a molecule and the number of peaks that molecule produces in carbon NMR and ask you to suggest its structure
To do this you have to use this method above ( trial and error) but you can speed up the process by comparing the number of peaks to the number of carbons in the molecule.
If the number of peaks is the same as the number of carbon in the molecule that suggests the molecule contains a carbon chain with not many branches
But if the number of peaks is lower than the number of carbons that suggest the molecule contains groups of carbon atoms
If analyzing the number of peaks on a NMR spectrum doesn't get us to an answer we can also use the positions of the peaks.
C-C
C-O
C=C
C=O
C-C give peaks of 0-50 ppm
C-O give peaks of 50-100 ppm
C=C give peaks of 100-150 ppm
C=O give peaks of 150 - 220 ppm
Which of these 4 molecules produce the Carbon NMR spectrum
Consider the number of peaks. There are 4 peaks so 4 different carbon environments. The 1st 3 molecules have 4 carbon environments. The last molecule has 2 carbon environments so we rule it out completely
Turn your attention to the positions of the peaks. This spectrum contains 2 peaks between 100-150 ppm so it must contains at least 2 carbon that bond like this C=C
The only option that satisfies that criteria is the first option
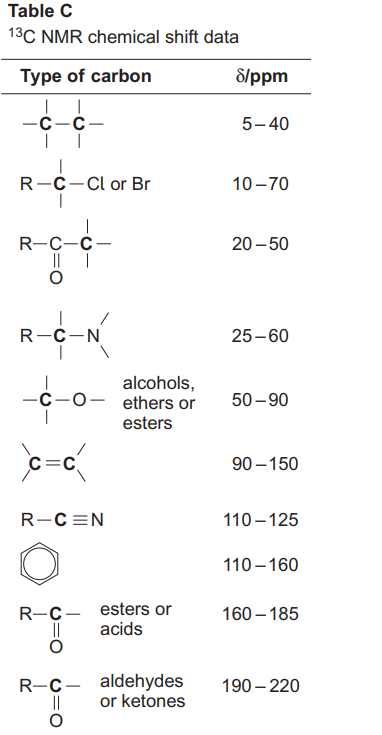
Specific shift tables
This is a more specific shift table (provided in the exams). For any given carbon we look at the atoms it is bonded to and that tells us which type it is. E.g if it is singly bonded to just carbons and hydrogen then it 5-40 type unless its i bonded directly to a carbonyl group which would mean it is 20-50 type
Remeber their are overlapping shift peaks E.g a sitf peak of 50 could be 4 different types of carbon we have to use process elimnation to work out which one it is
C-O (90) is alchohols esters or esters so it could be C-OH
Which of these molecules produced this spectrum
To answer this question first consider the number of peaks. This spectrum has 4 peaks so we can rule out option D because that option has 3 carbon environments
Next turn our attention to the positon of the peaks. The spectrum contains one peak at 175 ppm. If we look at the table we can see how the carbon should bond. Only option A has this bond.
how we would answer the question
in this question we got lucky with the peak we chose as it ended up being the defining which eliminated all other molecules this is unlikely. Sometimes we have to look at every single and slowly eliminate each molecule