Ap Chem 1/23
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
what is the goal for bonding
become stable (lowest energy) electron formation
share e-
covalent (NM+NM)
transfer e-
ionic (M+NM)
delocalized e-
Hot potato——are electrons that are not associated with a single atom or bond, allowing for resonance structures in molecules.
isomer
same formula but different arrangement of atoms
resonance
same formula same arrangement of atoms, different arrangement of electrons
what does VSEPR stand for ?
Valence Shell Electron Pair Repulsion Theory
what does VSEPR do
determines the shape of a molecule
what is hydrophilic
Polar
what is hydrophobic
Nonpolar
1 atom 0 L.P.
linear (180)
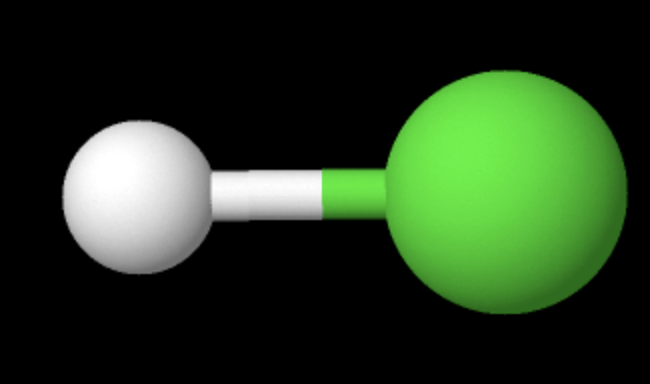
2 atom 0 L.P.
linear (180)
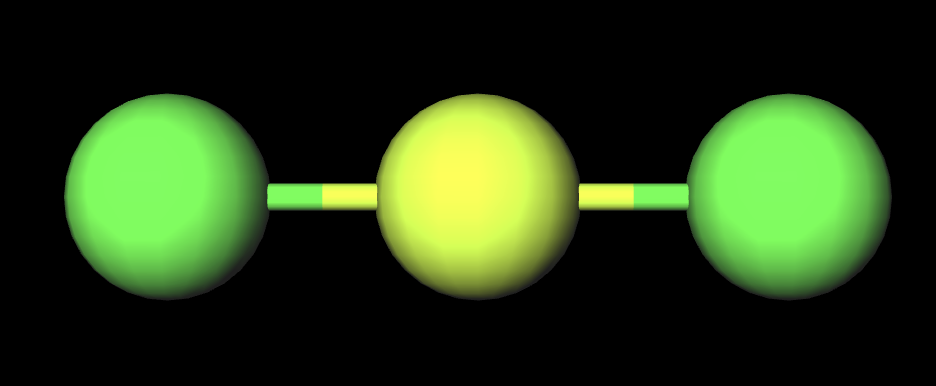
hybridization of linear structure(1atom 0LP)
sp
hybridization of linear structure(2atom 0LP)
sp
3 atoms 0 L.P.
trigonal planor (120)
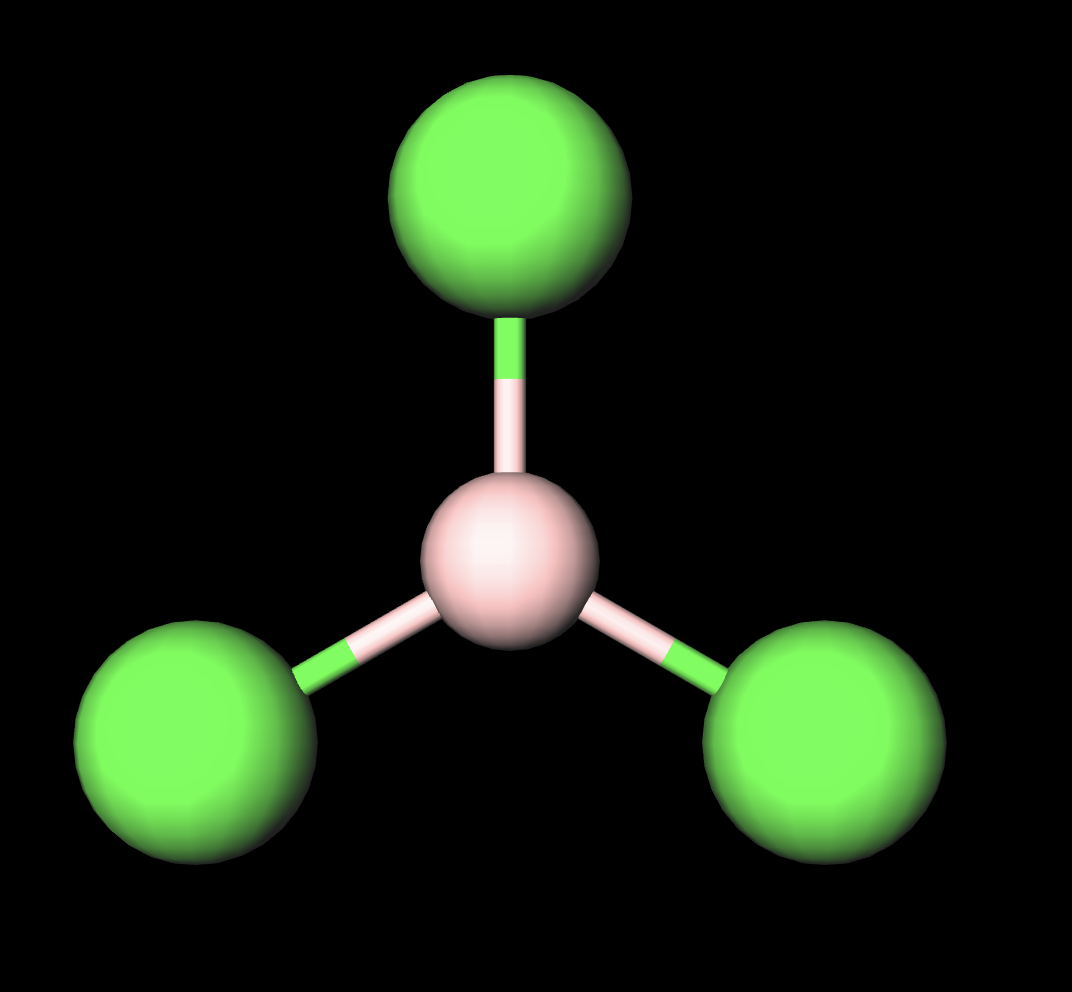
hybridization of trigonal planor structure(3atom 0LP)
sp2
4 atoms 0 L.P.
tetrahedral (109.5)
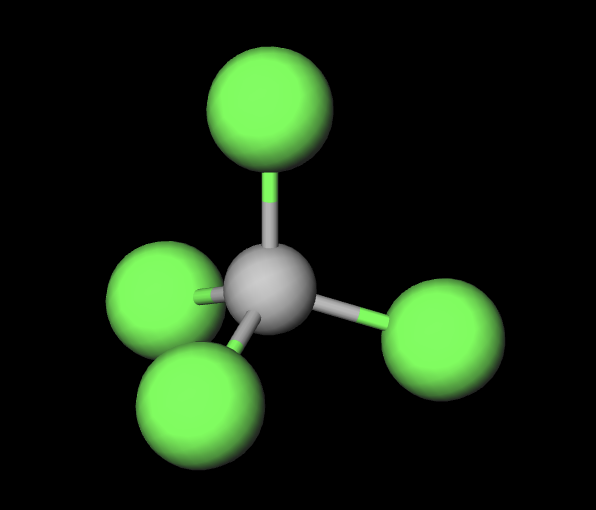
hybridization of tetrahedral structure(4atom 0LP)
sp3
3 atoms 1L.P.
trigonal pyramidal (107)
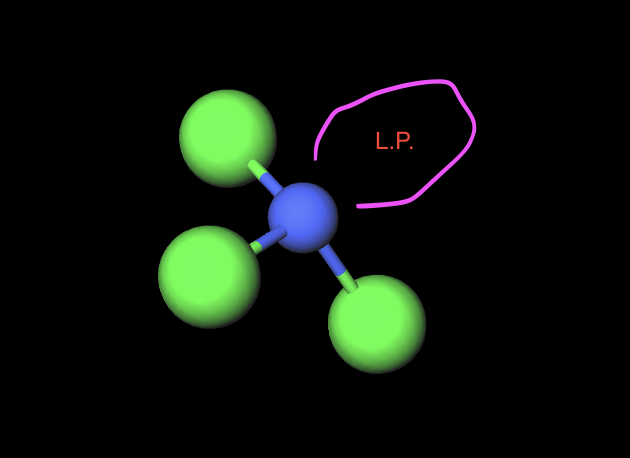
hybridization of trigonal pyramidal structure(3atom 1LP)
sp3
2 atoms 2 L.P.
Bent (104.5)
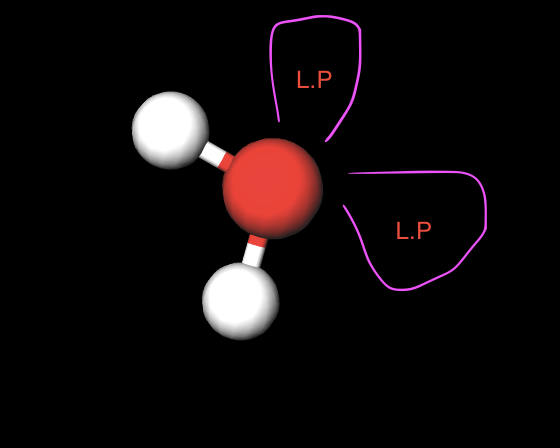
hybridization of a bent structure(2atom 2LP)
sp3
1 atom 3 L.P.
Linear (180°)
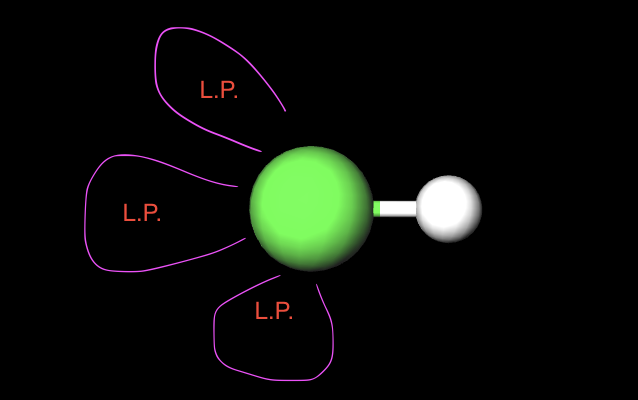
hybridization of a linear structure(1atom 3LP)
sp3
5 atoms 0 L.P.
trigonal bipyramidal (90 and 120)
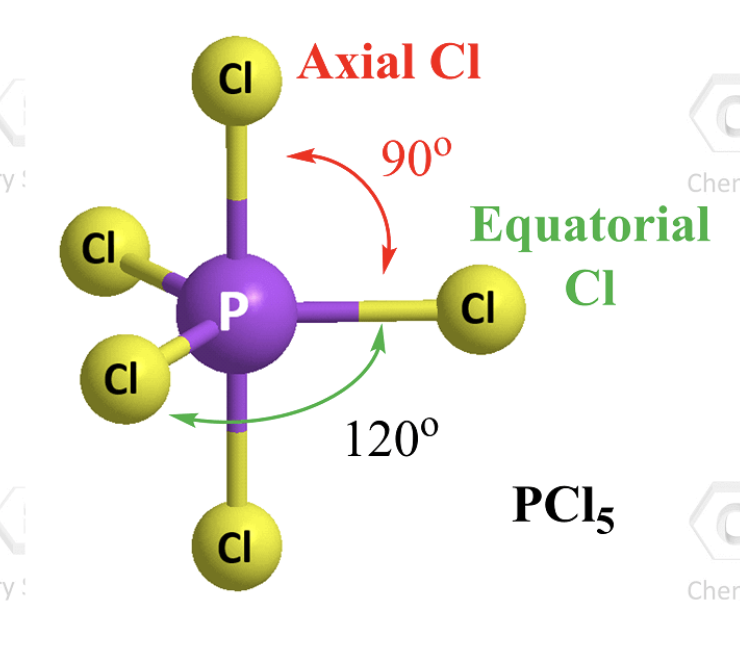
hybridization of a trigonal bipyramidal (5atom 0LP)
sp3d
6 atoms 0 L.P.
octahedral (90)
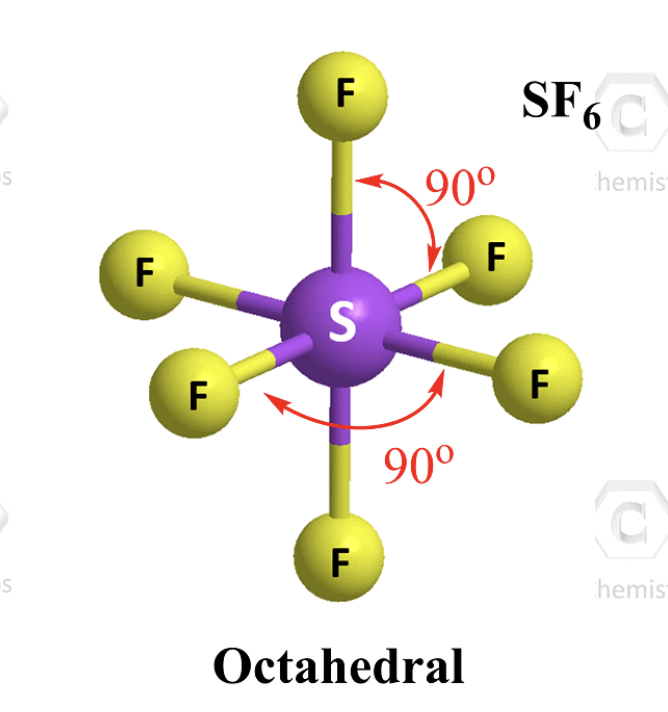
hybridization of octahedral structure (6 atoms 0 L.P.)
sp3d2
single bond
sigma
2nd or 3rd bond
Pi bond
what is the electron negativity difference of a polar bond
greater than or equal to 0.4
what is the covalent bond if the dipoles cancel out
non polar
is a linear structure polar or non polar
non polar
is a trigonal planor structure polar or non polar
non polar
is a octahedral structure polar or non polar
non polar
is a tetrahedral structure polar or non polar
non polar
is a by pyramidal structure polar or non polar
non polar
is a bent structure polar or non polar
polar
is a trigonal pyramidal structure polar or non polar
polar
what is an alloy
mixture of two or more metals
what type of bonding is an alloy
metalic bonding
what is an example of an alloy
brass
what two metals are in brass
copper and zync
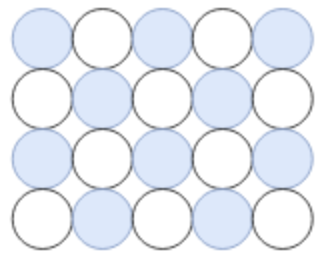
similar size alloy
substitute alloy
alloy that is made between on different size alloys
interstitial alloy
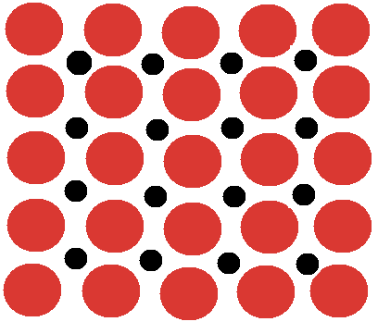
what type of alloy increases density
interstitial alloy
what is the electron C (ions)
hot potato *nothing wants to pick it up*
how does a substitute alloy work? example Cu+Zn
Zn substitutes where the Cu is