Chem 100 Final Study Guide: Comprehensive Review of Key Concepts and Terms
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
ion
An atom or group of atoms that has a positive or negative charge.
isotopes
atoms of the same element that differ in mass
fundamental unit of charge
measured in amu
molecular formula
a chemical formula of a molecular compound that shows the kinds and numbers of atoms present in a molecule of a compound
chemical symbols
abbreviations for the names of the elements
structural formula
a formula that shows the arrangement of atoms in the molecule of a compound.
empirical formula
a chemical formula showing the ratio of elements in a compound rather than the total number of atoms
average mass
fractional abundance x isotopic mass
Akali Metals
Highly reactive, group 1
alkaline earth metals
metallic elements in group 2 of the periodic table which are harder than the alkali metals and are also less reactive
physical properties
the characteristics of a substance that can be observed or measured without changing the substance
chemical properties
Characteristics of a substance that determine how it will react with other substances.
density
mass/volume
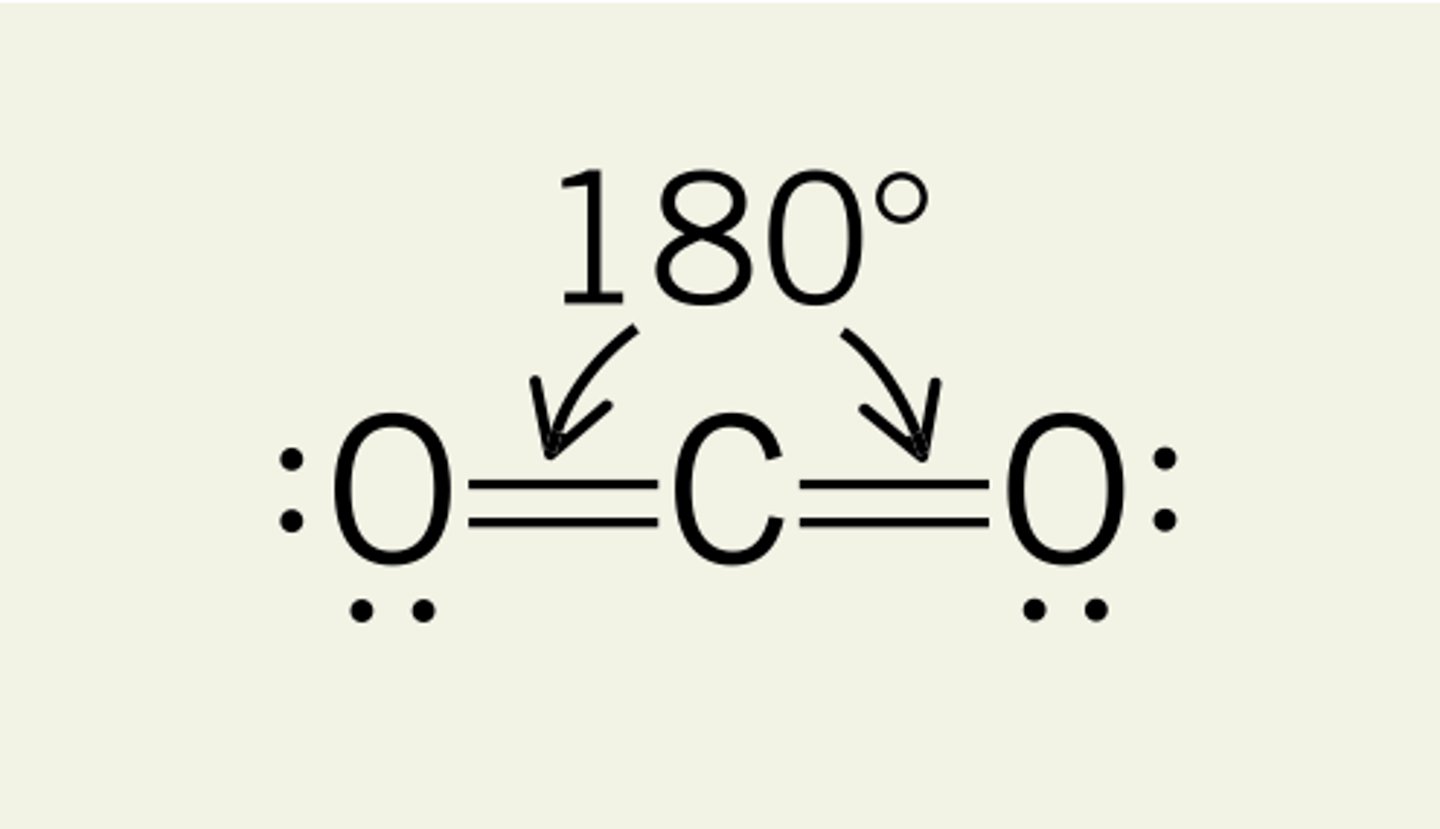
linear
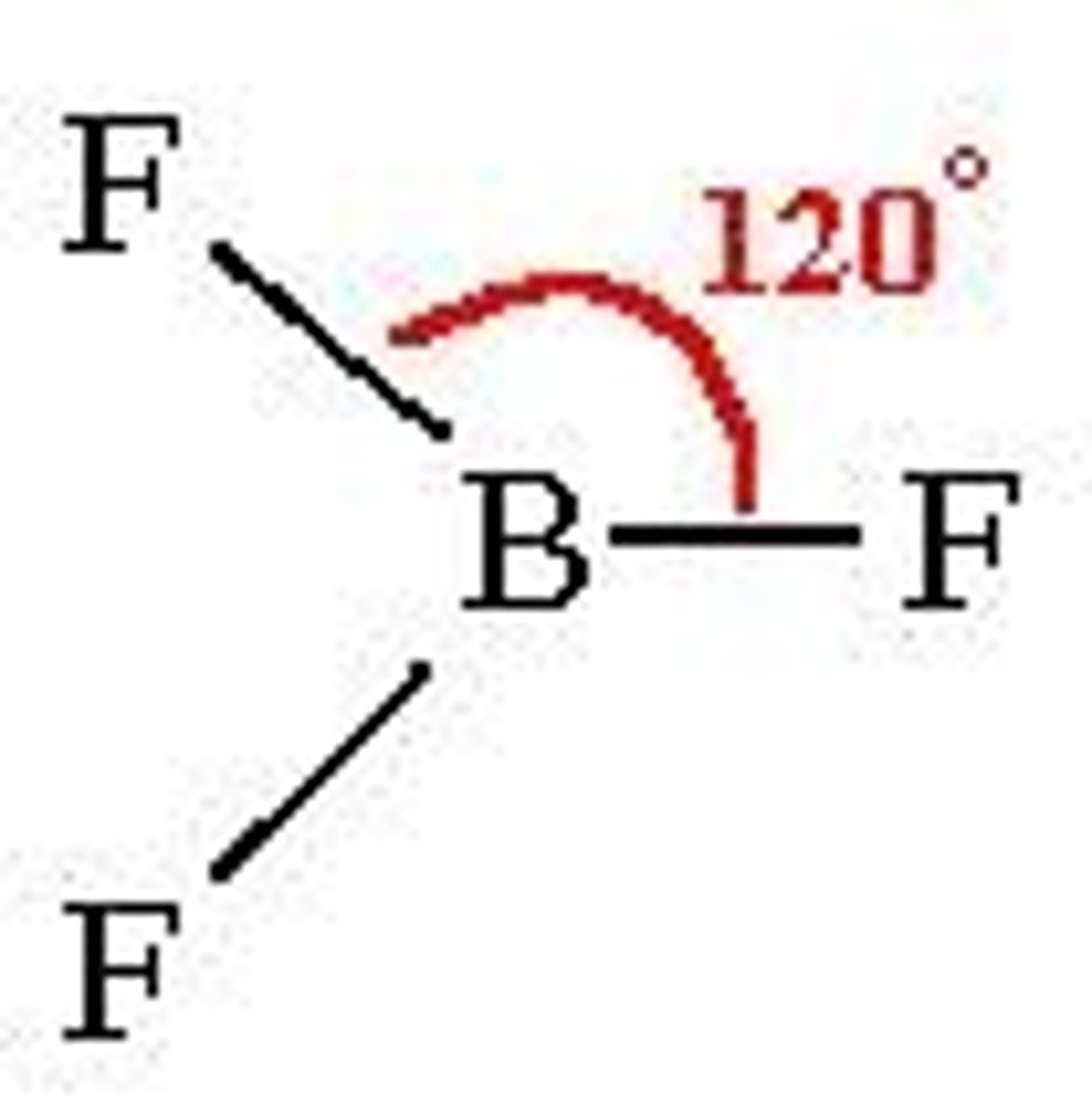
trigonal planar
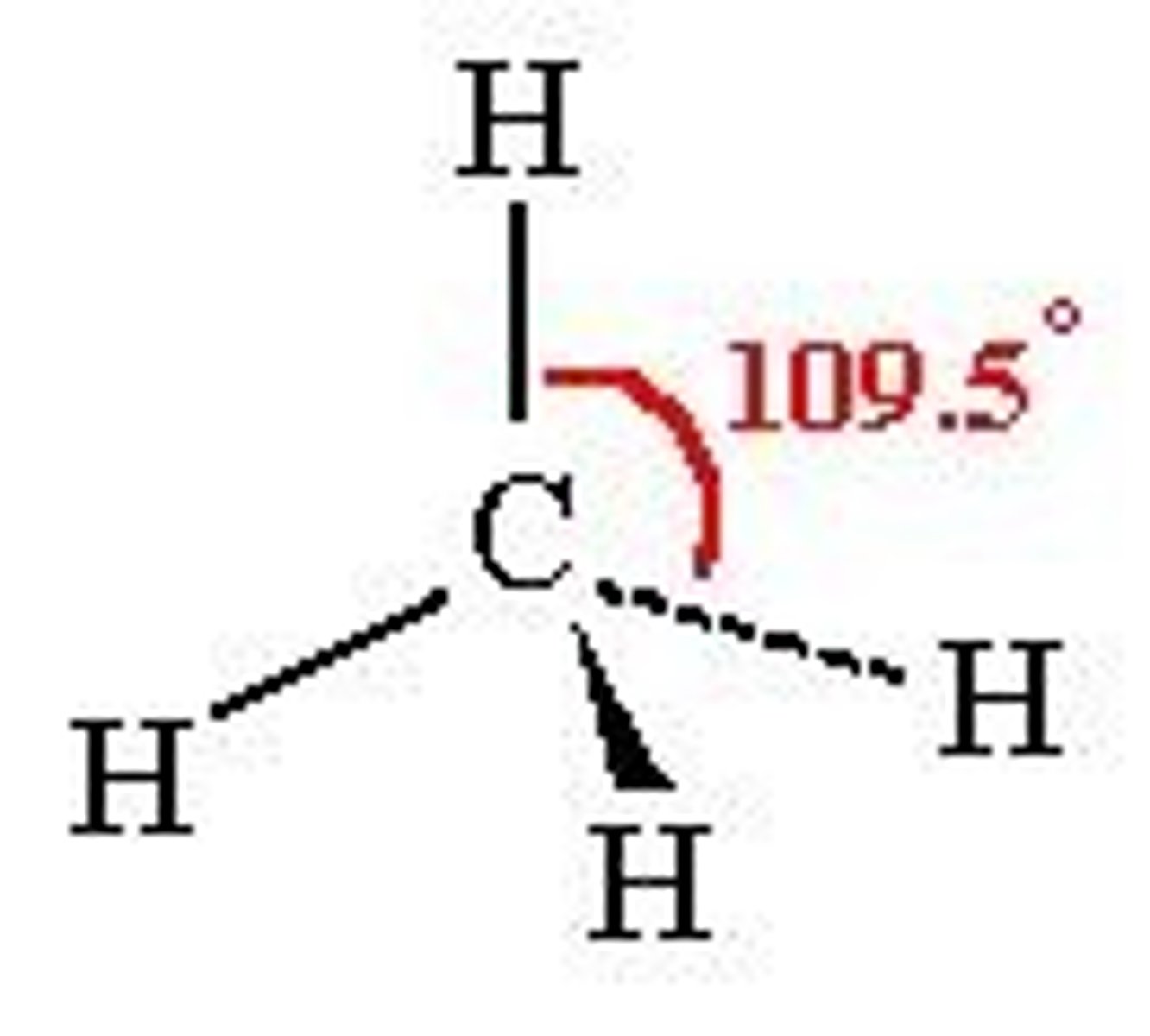
tetrahedral
109.5
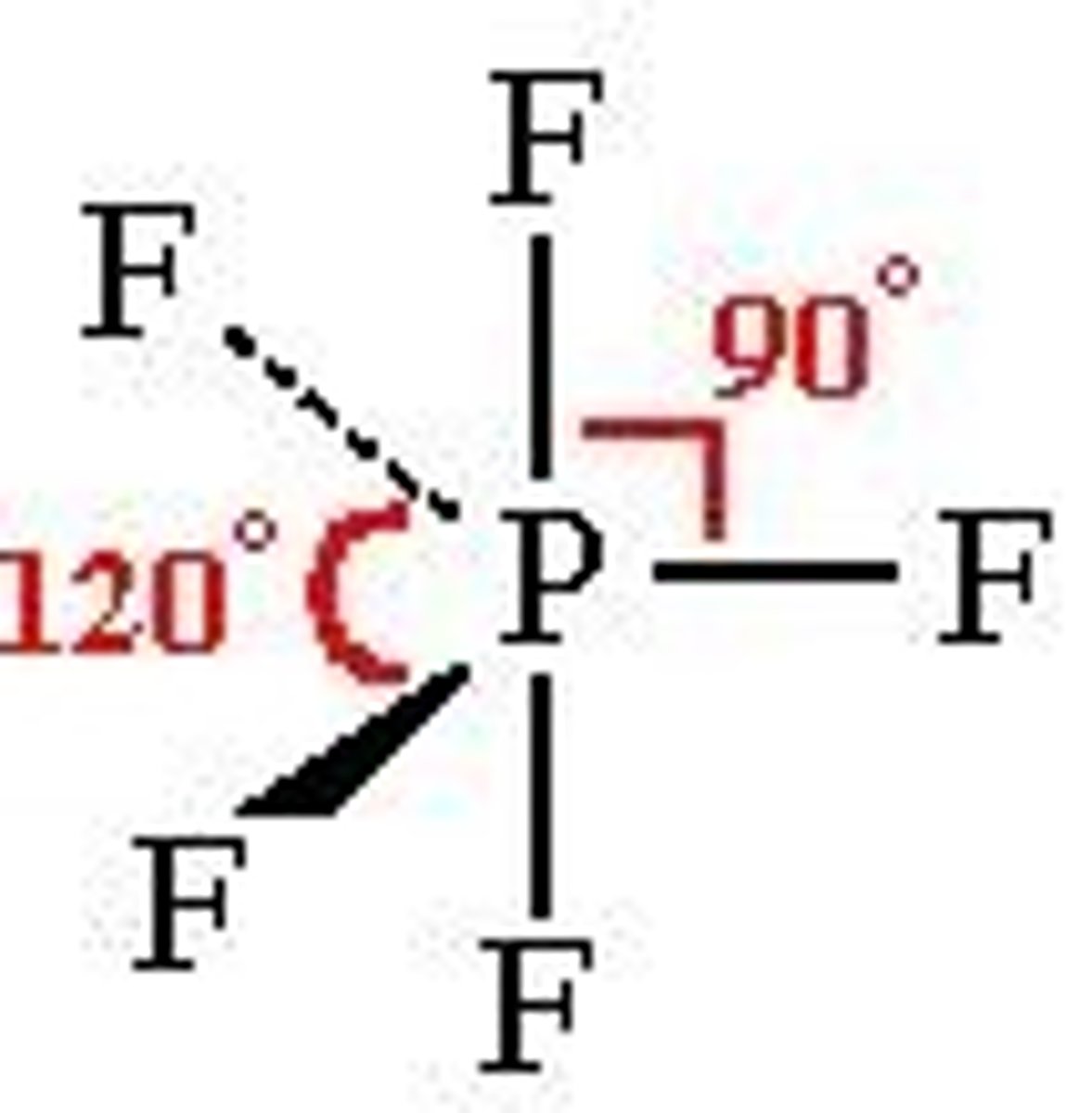
trigonal bipyramidal
5 bonds, 0 lone pairs
trigonal planar, bent
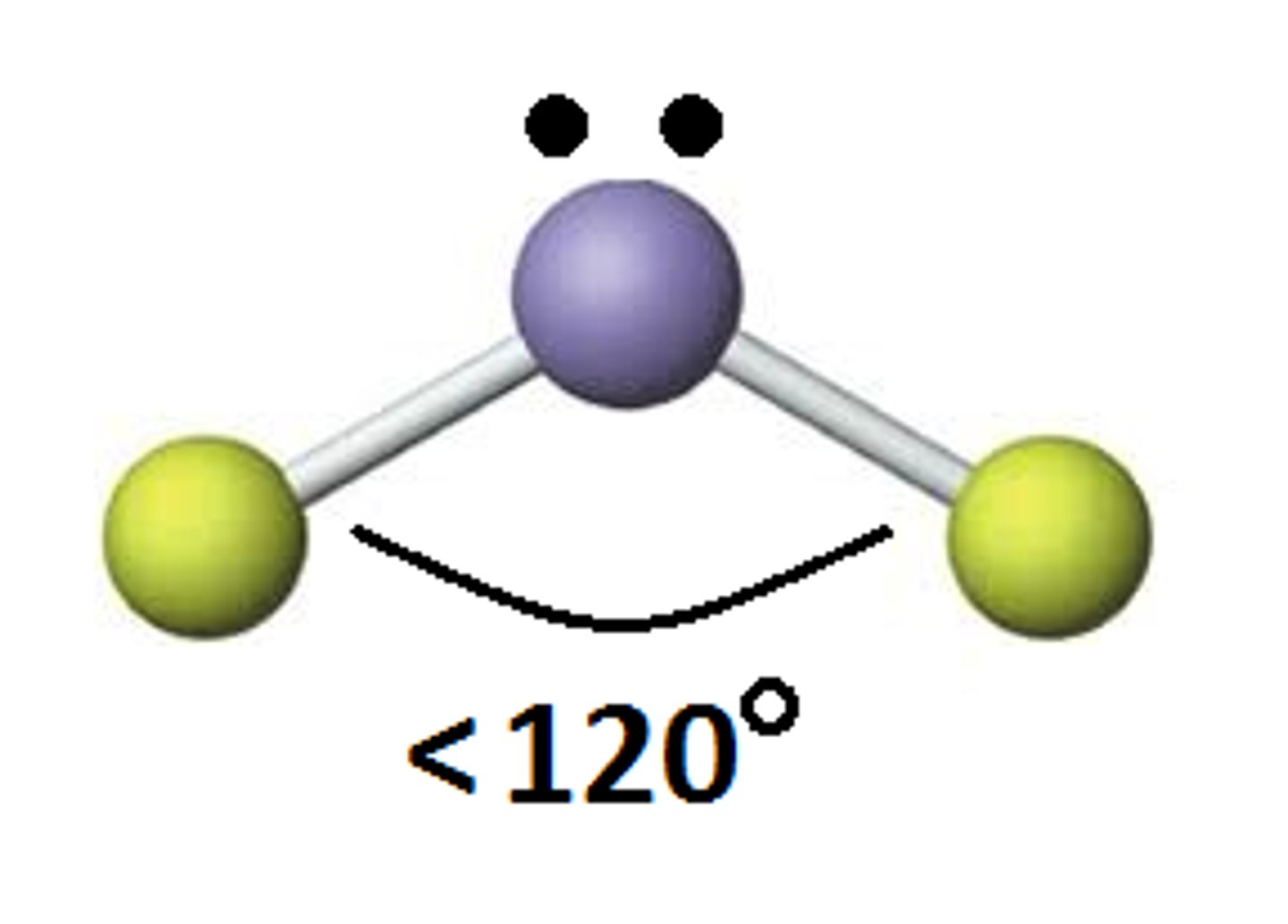
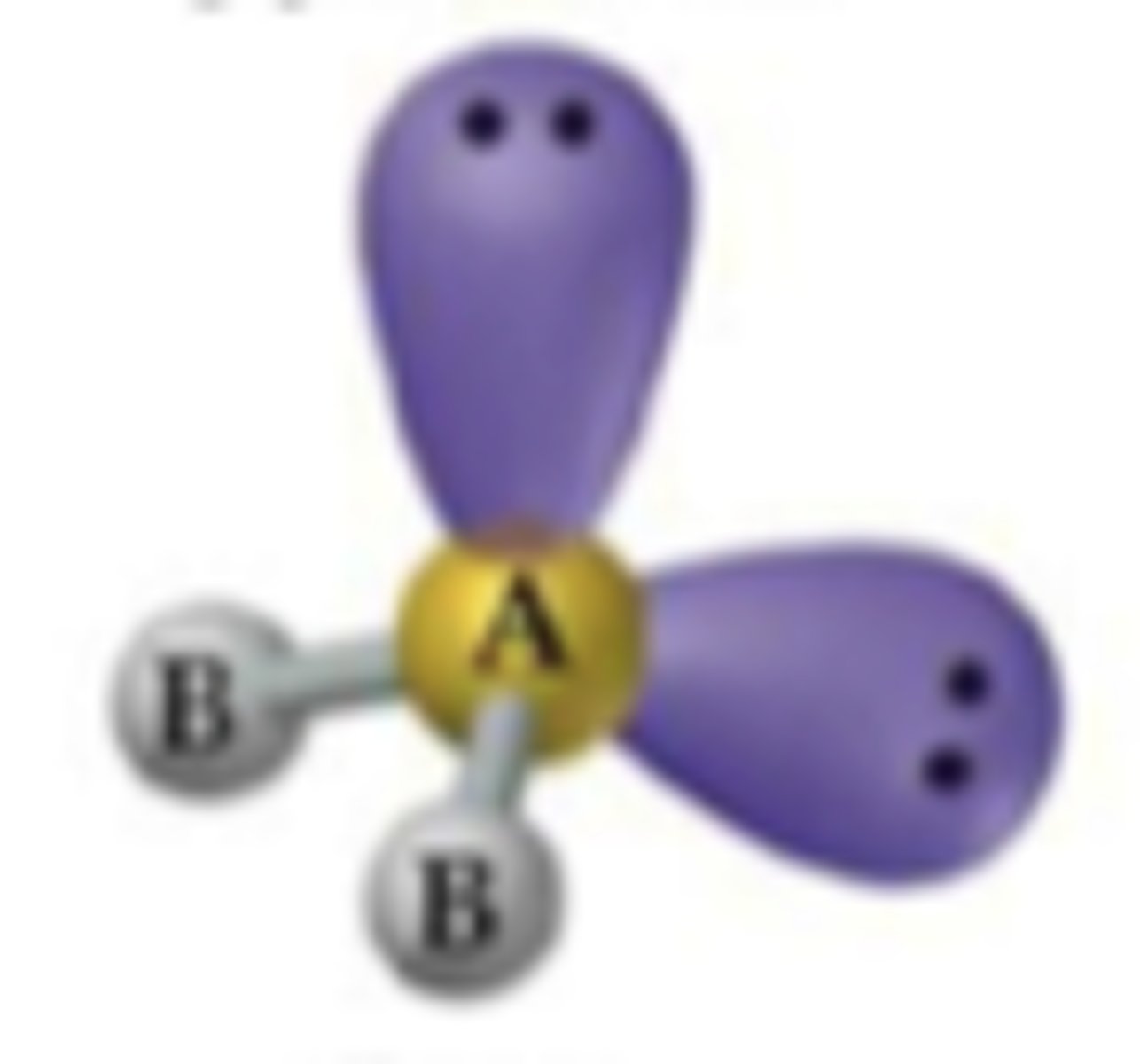
trigonal pyramidal
109.5
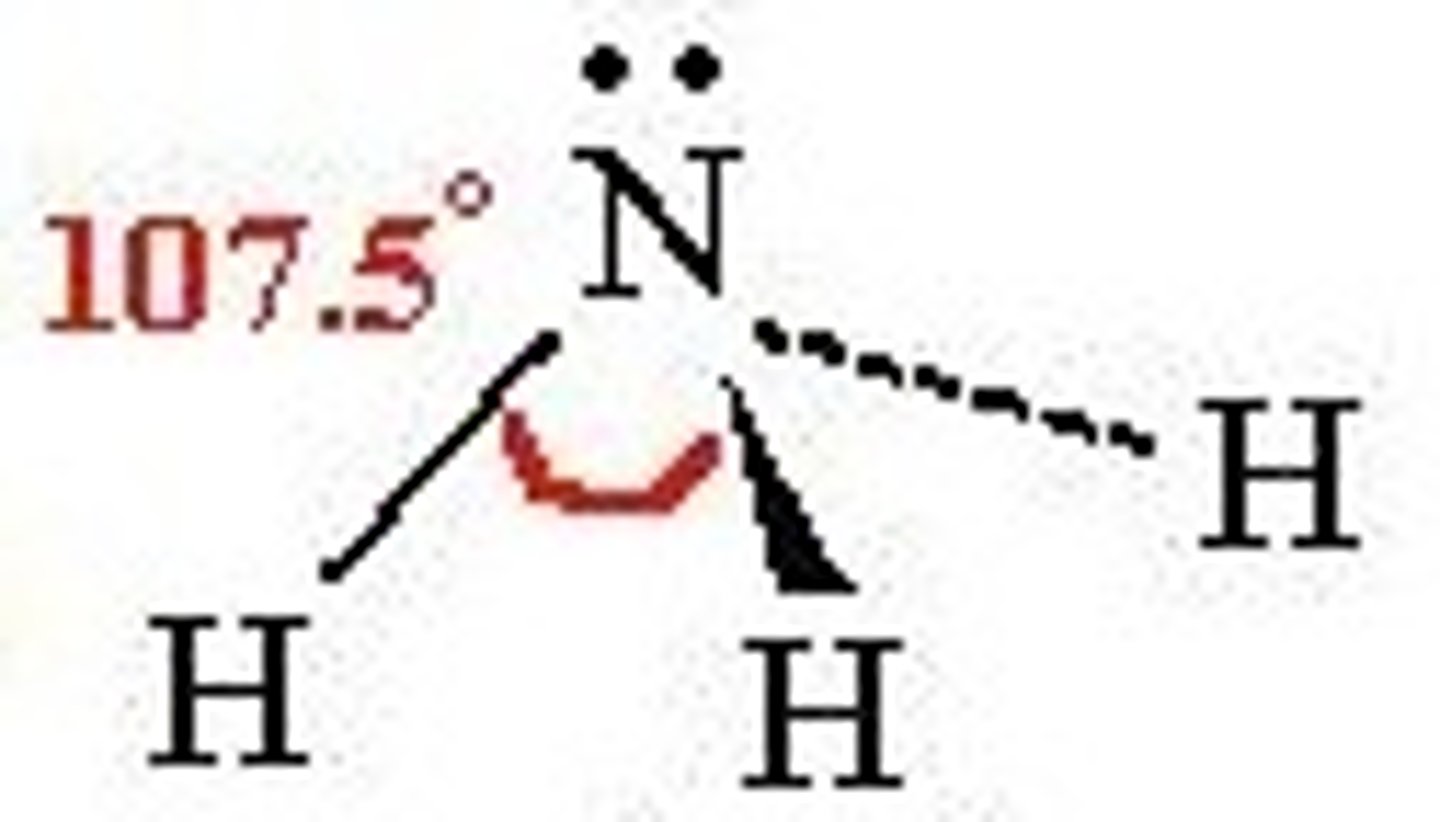
tetrahedral bent
109.5
Carbonate
CO3 2-
Bicarbonate
HCO3 -1
acetate
C2H3O2 -1
hydroxide
OH-
phosphate
PO4 3-
nitrate
NO3 -1
Sulfate
SO4 2-
PbO2
lead (IV) oxide
Binary Acids
composed of hydrogen and a nonmetal
Oxo Acid
H with a polyatomic ion in water
no hydro prefix
_______ate becomes ____ic acid
_______ite become _____ous acid
-ate
one more oxygen than -ite
polar molecule
0.5-2.0
nonpolar covalent bond
less than or equal to 0.4
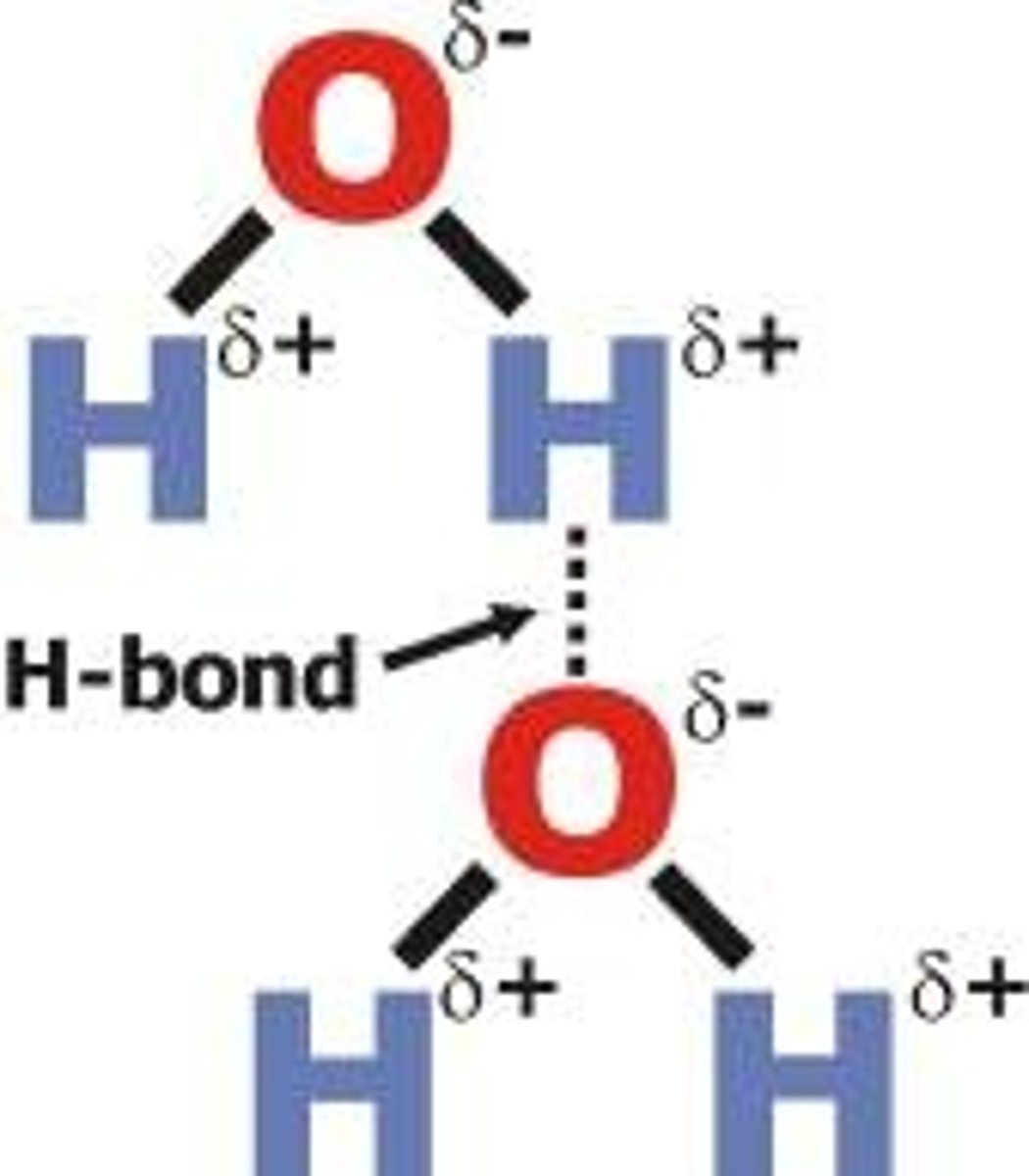
hydrogen bond
the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N, O, or F atom and another very electronegative atom.
how to calculate kelvin from celsius
Celsius + 273.15
what does R stand for in PV=nRT?
ideal gas constant; 0.08206 L-atm/mol-K