Wk 6: Infectious Diseases & Neoplasia
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
99 Terms
What are commensals?
Organisms that live symbiotically on or within the human host but rarely cause disease
What are opportunistic infections?
Infections resulting from commensals; Almost exclusively in immunocompromised hosts
What is colonization?
When symbiosis between commensal and host is disturbed, resulting in overgrown by either endogenous or exogenous organisms
What is a carrier?
Individual that harbors a specific microorganism
What is nosocomially acquired?
Hospital acquired
What is the difference between exogenous vs endogenous?
Exogenous: not normally found in or on body
Endogenous: one that is routinely cultured from a specific anatomic site, but that does not normally cause disease in the host
Which sites are normally sterile and any growth of bacteria is diagnostic of infection?
Blood, CSF, synovial fluid, or deep tissues of body
What are some mechanisms of innate (nonspecific) immunity?
Mechanical barriers
Chemical barriers (ex: enzyme action)
Natural killer cells (attack tumor cells)
Inflammation
Phagocytosis
Fever
What are is adaptive (specific) immunity and what are the cells that contribute to it?
AKA Immunity; Specialized lymphocytes that recognize foreign molecules in the body act against them
What is the difference between antigens vs antibodies?
Antigens (immunogens): Foreign substrates that can induce an immune response
Antibodies (immunoglobulins): Proteins that possess “specificity”, enabling them to combine with one particular antigenic structure.
What are the 3 things that occur when a pathogen breaks through mechanical and chemical barriers?
Macrophages try to phagocytize it
Activation of complement system
Dendritic cells present to B/T cells
What is the classical complement pathway?
Initiated by antigen-antibody complexes with the IgG and IgM antibody
Important byproducts include anaphylatoxins C3a, C4a, and C5a.
C3b and C4b facilitate opsonization
What is the alternative pathway?
Initiated by agents such as lipopolysaccharides (LPSs), trypsin-like molecules, IgA and IgG, and cobra venom.
Endotoxins are lipopolysaccharide (LPS) contained in the outer membrane of gram-negative bacteria.
Results in cell lysis and tissue inflammation
What are the 3 important steps of the complement cascades?
C3b and C4b facilitate opsonization
C5a recruits inflammatory cells
C5b-C9 result in cell lysis and tissue inflammation
What does deficiency of C3 lead to?
Leads to increase susceptibility to infections with encapsulated bacteria such as S. pneumonia and Haemophilus influenza
What does deficiency of C5-9 membrane attack complex lead to?
Leads to failure to lyse cells. As a result, these patients are susceptible to life-threating infections such as N. menigitidis and N. gonorrhoeae.
What are the 5 classes of antibodies and its functions?
IgG: provides majority of antibody-based immunity against pathogens. It responds later with the permanent eradication of antigen and its effect is lasting. It can cross placenta.
IgA: found in mucosal areas such as GI, respiratory, and urogenital tract. Also found in saliva, tears, and breast milk.
IgM: provides rapid adaptive immunity before there is sufficient IgG.
IgD: unknown
IgE: binds to allergens and triggers histamine release from mast cells. It is involved in allergy and protect against parasitic worms.
What is opsonization?
Opsonization is a process that helps your immune system identify and destroy old cells or germs (pathogens)
Antibodies binding to bacteria toxins or venoms may cause:
Neutralization
Antibodies may coat bacterial surfaces to:
Enhance phagocytosis through osponization
Antibodies may complex with antigens and:
Activate complement cascade, resulting in lysis of cell.
Antibodies can bind to NK cells then target cells and:
Release cytotoxins
What is the difference between antigen binding fragment (Fab) vs crystallizable fragment (Fc)
Antigen binding fragment (Fab) of AB recognizes the antigen, while crystallizable fragment (Fc) of AB interacts with other elements of the immune system, such as phagocytes or components of the complement pathway.
What are the potential outcomes of infection?
Resolution
Chronic infection with host tissue damage (Parasitic)
Ex: HIV or hepatitis B (human host is infectious for the virus with progressive liver damage and cirrhosis)
Chronic infection w/o host tissue damage (Saprophytic)
Ex: carrier state with Salmonella typhi, or hepatitis B (human host is infectious for the virus but has no clinical evidence of liver damage)
Chronic infection with possible reactivation (Latency)
Ex: varicella zoster virus
Host death from infection
What is infective endocarditis and what are the main sites of infection?
A bacterial infection of the cardiac valves. Patients with structurally abnormal valves are at increased risk.
MC site: mitral and aortic valves
What infectious agents cause infective endocarditis?
MC infectious agents are gram-positive bacteria including:
Viridans streptococci: recent dental work (MC)
Staphylcoccus aureus: nonsterile needles introduce skin bacteria
Enterococci: genitourinary tract infections
What is the pathogenesis of infective endocarditis?
Bacteria adhere to fibrin-platelet clots that form at the site of damaged cardiac endothelium.
The fibrin-adherent bacteria activate monocytes to produce mediators such as cytokines and platelet tissue factor, resulting in further recruitment of platelets and growth of the vegetation.
This provides the bacteria a sanctuary from host defense mechanism such as leukocytes.
What are the clinical manifestations of infective endocarditis?
Murmur when auscultating the heart sound
Oslar nodes: painful nodules on fingers and toes
Janeway lesions: painless hemorrhagic macules on palms and soles
Splinter hemorrhages: nail beds
Roth spots: red spots with white centers d/t deposition of immune complex or septic micro-emboli.
***THIS WILL BE ON EXAM
What is the difference between bacterial vs viral menigitis?
Bacterial meningitis causes significant morbidity and mortality thus requires immediate antibiotic therapy.
Viral meningitis are often self-limiting and more common.
How do we diagnose meningitis?
Culture of CSF
What is the most common bacterial pathogen of meningitis?
S. pneumoniae and N. meningitidis
What is the most common viral pathogen of meningitis?
Herpes simplex and Non-polio enterovirus (coxackie)
What is the pathogenesis of meningitis?
Pathogenic bacteria first adheres to and colonizes the nasopharynx.
They secrete an IgA protease that inactivates host antibody.
Once in the bloodstream, the bacterial capsule helps evade opsonization.
The bacteria then accesses CSF through receptors on the endothelial surface of the blood-brain barrier.
What are the clinical manifestations of meningitis?
Most patients develop rapid onset of lethargy, fever, headache, photophobia, neck stiffness, nuchal rigidity.
Kernig sign: resistance to passive extension of the flexed leg with patient laying supine
Brudzinski sign: involuntary flexion of hip and knee when examiner passively flex’s the patient’s neck
***THIS WILL BE ON EXAM
What is the leading cause of death from an infectious disease in the US and which population does it disproportionally affect?
Pneumonia, together with influenza
Disproportionally a disease of elderly and impaired host
What is pneumonia?
Infection of the lung tissues caused by a number of bacteria, viruses, parasites, and fungi, resulting in inflammation of lung parenchyma and accumulation of an inflammatory exudate in the airways.
What is the most common bacterial and viral cause of pneumonia?
MC viral cause: Influenza (flu)
MC bacterial cause:bCommunity-acquired pneumonia: S. pneumoniae, Nosocomial-acquired pneumonia: MRSA
What is the pathogenesis of pneumonia?
Abrupt changes in direction of airflow in the nasal passage can trap potential pathogens.
The epiglottis and cough reflex prevent introduction of particulate matter in the lower airway.
The ciliated respiratory epithelium propels the overly mucus (lysozyme and IgA) layer upward toward the mouth.
In the alveoli, cell-mediated immunity (CD4 and CD 8 T lymphocyte), humoral factors (IgA), and inflammatory response (macrophage, neutrophils, complement) defend against lower respiratory tract infection.
Impairment at any level of host defense increases the risk of developing pneumonia.
What are the clinical manifestations of pneumonia?
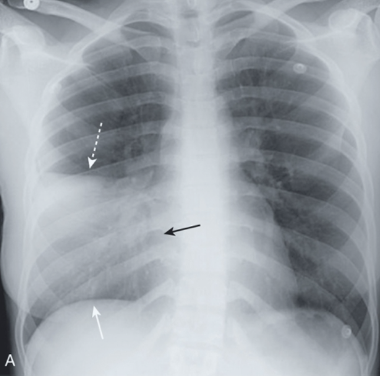
Fever, cough, tachypnea, tachycardia, asymmetric ↑ tactile fremitus (vibration), dullness on percussion, consolidation on chest x-ray film (see picture attached)
GI tract infections can present with primarily:
Upper tract S/S: nausea, vomiting, cramping epigastric pain
Small intestine S/S: profuse watery diarrhea
Large intestine S/S: fecal urgency, bloody diarrhea
Sources of infectious diarrhea include:
Person-person: fecal-oral spread of Shigella
Water-borne: Cryptosporidium
Food-borne: Salmonella or S. aureus food poisoning
Returning traveler: E.coli (Enterotoxigenic)
Overgrowth after antibiotic administration: Clostridium difficile
What are the three types of infectious diarrhea?
Secretory (watery) Diarrhea: Called “rice water stool” d/t milky white color
Inflammatory Diarrhea
Hemorrhagic Diarrhea
What is the pathogenesis of secretory (watery) diarrhea?
Caused by ETEC (enterotoxigenic E. coli) and EAEC (enteroaggregative E. coli)
They produce enterotoxins, which causes prolonged activation of epithelial adenylyl cyclase in the small intestine, leading to secretion of massive amounts of fluids and electrolytes
What is the pathogenesis of inflammatory diarrhea and its S/S?
Caused by Shigella, Salmonella, EIEC (enteroinvasive E. coli)
Result of bacterial invasion of mucosal lumen. The production of cytotoxin (Shiga toxin or Shigella-like toxin) leads to cell death.
S/S: dysentery (↑ fecal leukocytes and bloody diarrhea with mucous)
What is the pathogenesis of hemorrhagic diarrhea?
A variant of inflammatory diarrhea + hemolytic uremic syndrome (anemia + renal failure)
Caused by EHEC (enterohemorragic E. coli), associated with E.coli O157:H7
The bacterial releases Shiga toxin that catalyzes the destructive cleavage of ribosomal RNA and halts protein synthesis, leading to cell death.
If diarrhea is accompanied by fever, bloody stools, or abdominal cramping, what test should we order?
Stool sample
What is sepsis?
SIRS (systemic inflammatory response syndrome) + an infectious precipitant.
What is the difference between severe sepsis vs septic shock?
Severe sepsis = sepsis + organ dysfunction
Septic shock= sepsis + hypotension unresponsive to fluid resuscitation
What is the main cause of sepsis?
Although evidence of infection is a diagnostic criterion for sepsis, only 28% have bacteremia. MC: Staphylococci
What is the pathogenesis of sepsis?
Sepsis generally starts with a localized infection
Bacteria then invade the bloodstream and proliferate locally and release toxins (endotoxins and exotoxins) into the bloodstream
Eventually leads to multiple organ dysfunction
What are the difference between endotoxins vs exotoxins?
Endotoxins: lipopolysaccharide (LPS) contained in the outer membrane of gram-negative bacteria.
Exotoxins: proteins synthesized and released by bacteria
What are the clinical manifestations of sepsis?
Tachycardia, tachypnea, alterations in temperature and leukocyte count
Microvascular injury → poor tissue perfusion and cellular hypoxia→ ↑ lactic acidosis
What tests should you run and actions to take when suspecting sepsis?
Obtaining culture of blood and body fluid, fluid resuscitation, administrate broad-spectrum antibiotics
In addition to mutational changes that alter the genetic code, what changes also underlie cellular and biochemical aberrations that contribute to the malignant phenotype?
Epigenetic changes
What do methyl groups and histones do?
Methyl group (DNA methylation) acts like a switch to the gene expression, while histone (histone modification) acts like a knob of the gene expression.
Tumor initiation starts with development of:
Defects in the genes involved in the machinery that guards the genome.
What are tumor suppressor genes?
Genes that regulate normal cell division and replication.
Include proteins involved in DNA damage control, cell cycle control, programmed cell death, and cell adhesion.
Several mutations (ex: nonsense mutations, frame-shift mutation, or deletion mutation) can inactivate these genes, resulting in loss of the function alterations (loss of the protein product of the gene)
More common
Many are hereditary
Ex: p53, BRCA1/BRCA2, PTEN, APC
What is the p53 gene and what does it do?
Tumor supressor gene that recognizes DNA damage and induce apoptosis.
Loss of p53 can result in continued cell replication despite DNA damage and failure to activate apoptosis
What is the PTEN gene and what does it do?
Tumor suppressor gene produces phosphatase protein that is involved in the regulation of cell cycle and preventing cells from growing or dividing too quickly.
Loss of PTEN function results in increased cell proliferation and reduced cell death
What is the Cadherins (CDH) gene and what does it do?
Tumor suppressor gene that produce proteins (cadherins) involved in cell-cell adhesion.
Loss of cadherins result in cell detachment and metastasis.
What are oncogenes?
Genes that have the potential to cause cancer.
Mutation, gene amplification, or overexpression can activate these genes, resulting in gain of function alteration.
Oncogenes can also be acquired through introduction of foreign genomic materials, usually virus.
Ex: EGFR/HER1, HER2/Neu, B-Raf
What are the EGFR/HER1 genes and what do they do?
Oncogene that signal proliferative and apoptotic pathways.
Overactivity of EGFR/HER1 genes lead to unregulated control of growth and apoptotic signaling.
What is the Ras gene and what does it do?
Oncogene that can switch on other proteins, resulting cell growth/differentiation/survival.
Mutational activation of Ras causes unintended and overactive of signal
What role do the growth factor receptor tyrosine kinases (RTKs) play?
Plays an essential role in cell proliferation and growth. They have a significant effect on cancer progression, and they can be mutated, amplified, or normal but increased expression.
What role do the transforming growth factor–β (TFG- β) play?
Inhibit cell proliferation but stimulates production of ECM and adhesion factors. In some tumor type, oversecretion of TFG- β promotes invasive and metastatic property of tumor.
How does estrogen play a role in neoplasia?
Estrogen are important in the development of breast cancer. Estrogen receptor, although no abnormalities are found, is essential throughout breast cancer development.
What is carcinoma?
Originate from ectodermal or endodermal tissues (MC type).
Ex: epithelial tissues cancers such as lung, colon, breast, prostate
What is sarcoma?
Originate from mesodermal tissues.
Ex: connective tissues predominantly
Spectrum of neoplastic phenotype of epithelial cells range from:
Hyperplastic: Can be dysplasia or metaplasia
Preinvasive (Carcinoma in situ): Aggressive proliferation without invading through basement membrane
Invasive: Aggressive proliferation with the breach of basement membrane
Metastatic: Proliferation through lymphatic system or bloodstream to distance organs or other tissues
What is the general cause of colon cancer?
Mostly due to sporadic mutation, some due to known mutation.
What causes familial adenomatous polyposis?
Result of a mutation in the APC gene, which results in abnormal cell proliferation
What causes hereditary nonpolyposis colorectal cancer?
Associated with mutations in DNA repair genes hMSH2.
What are the steps of genetic alterations in colon cancer development?
Mutation in APC gene → abnormal proliferation
Mutation in K-ras gene → abnormal growth and differentiation
Mutation in p53 gene → continue cell replication despite DNA damage
Mutation in hMSH2 → no DNA repair resulting in chromosomal instability
Mutation in DCC gene → abnormal cell growth and apoptosis
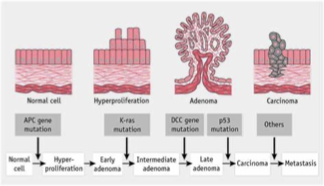
What kind of substances can be mutagenic in regards to colon cancer?
Substances derived from bacterial colonic flora, ingested foods, or endogenous metabolites such as benzopyrenes found in coal tar and cigarette smoke are mutagenic.
Hematogenous spread from colorectal tumors to which body parts are common?
Liver, lungs, bone
What are the risk factors of colon cancer?
Cigarette smoking
Diet high in red meat
Diet low in fibers
Obesity
Elderly
Male
IBS
What are the diagnostic tests or imaging can we order when suspecting colon cancer?
Colonoscopy and fecal occult blood
What are the genes that contribute to the development of breast cancer?
Mutated BRCA1 and BRCA2 → abnormal DNA repair
Overexpression of HER1 gene / HER2 gene → abnormal cell proliferation
Mutated P53 → continue cell replication despite DNA damage
What is the difference between ductal carcinomas vs lobular carcinomas?
Ductal carcinomas: Most common breast cancer; Cancers arising from lactiferous duct
Lobular carcinomas: Cancers arising from terminal lobules without crossing basement membrane and invade ducts. Loss of cell adhesion protein Cadherin thus grow more diffusely
What are the risk factors of breast cancer?
Prolong usage of high doses of exogenous estrogen
↑ numbers of menstruation (early age menarche and late age menopause)
Ionizing radiation
Early pregnancy and longer time breast feeding ↓ risks
What are the s/s of breast cancer?
Hard, painless lump (common in upper and outer breast)
Dimpling of skin
Swelling under armpits
What tests and actions can you take when suspecting breast cancer?
Breast exam and mammogram
What accounts for a large proportion of tumors of childhood?
Neuroendocrine, germ cell neoplasia, and sarcoma
What is the common first sign of testicular germ cell cancers?
Painless testicular enlargement
Hematologic neoplasms are malignancies of cells derived from:
Hematopoietic precursors
Hematologic malignancies are often linked to certain:
Chromosomal translocations
Mutations or deletions of what genes and activation of what oncogene are commonly seen in hematologic neoplasms?
Mutation or deletion of p53 & RB gene, activation of Ras
What is lymphoma?
Cancer (mass) of the lymphatic system derived from the immune system, which results from neoplastic proliferation of B (MC) or T lymphocytes.
What is Hodgkin lymphoma?
Better prognosis
Presence of Reed-Sternberg giant cell is the characteristic feature
Many are associated with Epstein-Barr virus (EBV)
HIV is a risk factor
What is Non-Hodgkin Lymphoma?
Most common, included all types of lymphoma except for Hodgkins
Majority involve B cells, few are of T cell lineage
Infectious agents include Epstein-Barr virus, Helicobacter pylori, Hepatitis C, and HIV.
Chemicals such as phenoxy herbicides, radiation therapy or chemotherapy, autoimmune disease, are all possible causes
What is the pathophysiology of non-Hodgkin lymphoma?
Most non-Hodgkin lymphoma exhibit chromosomal translocation, resulting in increased expression of the oncogene product.
MC: t(8;14), t(14;18), t(11:14)
How are leukemias classified?
By the lymphocytic or myelocytic linage as well as acute (↑ blast cells with no matured cells) or chronic (cells mature partially thus do not work effectively)
What is Acute lymphoblastic leukemia (ALL)?
A rapidly progressive neoplasm derived from lymphoblasts, which overtake the bone marrow and can infiltrate other organs like brain and liver.
Philadelphia chromosome can be seen in some cases.
What is Acute myelogenous leukemia (AML)?
Most common acute leukemia.
It involves bone marrow and usually manifest abnormal circulating blast cells. These blast cells normally develop into white blood cells. However, in AML, these cells do not develop and are unable to ward off infections.
Chromosomal translocation t(15;17) had been noted.
Hallmark is Auer rods (large crystalline cytoplasmic inclusion bodies)
What is Chronic myelogenous leukemia (CML)?
Begins in the blood-forming cells of the bone marrow and then, over time, spreads to the blood.
Hallmark is the presence of Philadelphia chromosome, t(9;22) and increased granulocytes and monocytes.
Genetic testing for conclusive DX.
What is Chronic lymphocytic leukemia (CLL)?
Begins in lymphocytes in the bone marrow and extends into the blood.
Can also spread to lymph nodes and organs such as the liver and spleen.
CLL develops when too many abnormal lymphocytes grow due to chromosomal defect, crowding out normal blood cells and making it difficult for the body to fight infection.
Hallmark is smudge cells (immature B cells break during the smear).
Genetic testing for conclusive DX.
What is a sign that is present in all subtypes in non-Hodgkins Lymphoma?
Hepatosplenomegaly and lymphadenopahy
What is the unique cytochemistry marker present in acute myelogenous leukemia (AML)?
Myeloperoxidase
What is the unique cytochemistry marker present in acute lymphocytic leukemia (ALL)?
TDT (terminal deoxynucleotidyl transferase)