Chemistry Exam 4
0.0(0)
Card Sorting
1/113
Last updated 9:45 PM on 5/1/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
1
New cards
Pretend that you are working in a lab group. **If your grade on a laboratory depended on how accurately and precisely your team measures the length of a sheet of paper, what would be some of your team's concerns about your group’s measurement?**
Group gets the right measurement, Everyone in group gets the same answers, everyone uses an accurate ruler, and everyone uses the same type of measuring units (Like inches, centimeters, etc.)
2
New cards
How would your team determine which measurement would be the one reported?
Take the average of the group’s measurement
3
New cards
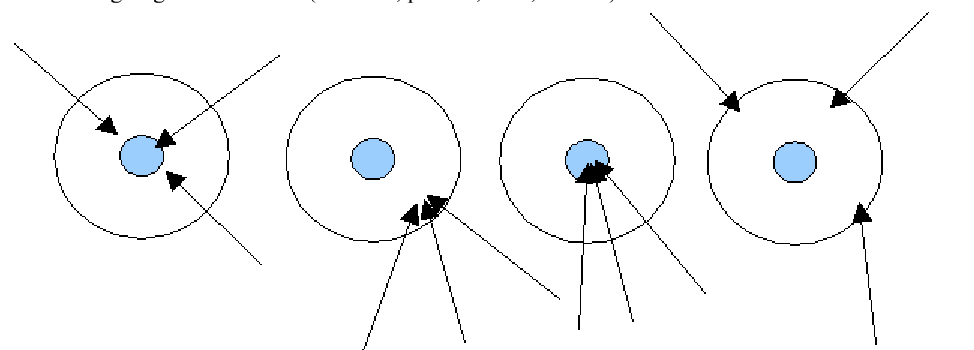
Which of the following describes the first target?
Accurate
4
New cards
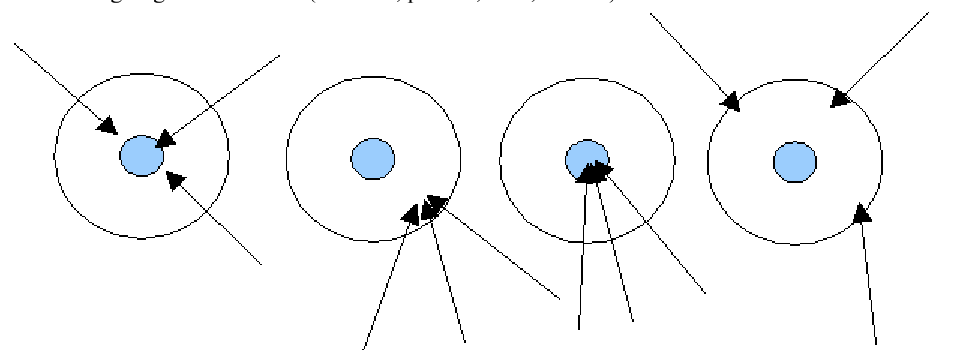
Which of the following describes the second target?
Precise
5
New cards
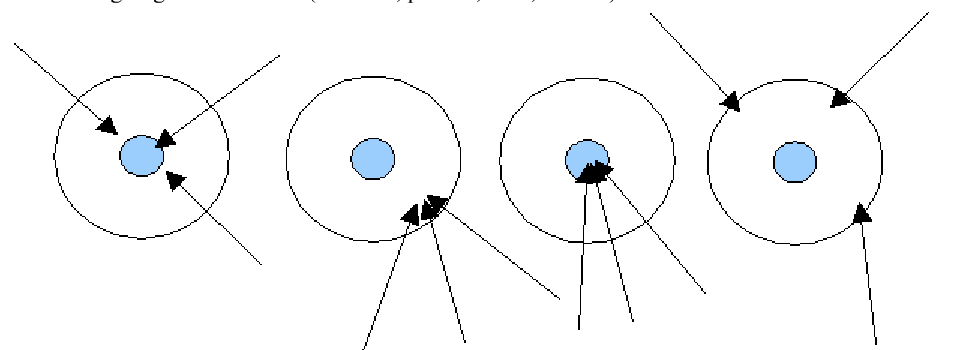
Which of the following describes the third target?
Both
6
New cards
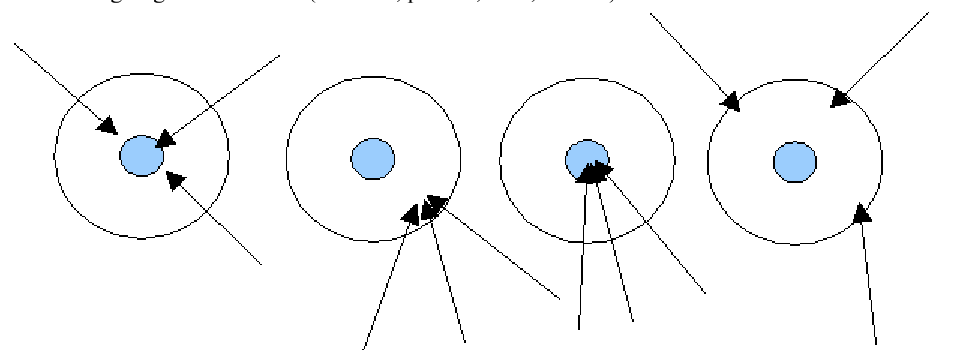
Which of the following describes the fourth target?
Neither
7
New cards
Which of the following is a simple explanation for accuracy?
Accuracy means getting the right answer
8
New cards
Which of the following is a simple explanation for precision?
Precision means getting the same answer every time
9
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.08 is..
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.08 is..
Accurate
10
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you chose this answer for the 0.08% reading?
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you chose this answer for the 0.08% reading?
It is close to the true blood alcohol concentration of 0.0764% and it is not precise since it only measures to 0.08 Instead of 0.0764
11
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.1 is?
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.1 is?
Neither
12
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you choose this answer for 0.1% reading?
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you choose this answer for 0.1% reading?
This answer is not accurate because it indicates that the person was well over the legal limit of 0.08% when the person was actually slightly below it and it is not precise since it only measures to one decimal place instead of 4 decimal places like the true reading.
13
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.076% is?
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.076% is?
Both (accurate and precise)
14
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you choose this answer for 0.076%.
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you choose this answer for 0.076%.
The answer is accurate compared to the true value and precise since it measures to 3 decimal places.
15
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.0504 is?
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
The reading of 0.0504 is?
Precise
16
New cards
Determine if the following readings from a breath analysis for blood alcohol are accurate, precise, both, or neither for a person arrested with a true blood alcohol concentration of 0.0764%. Recall in most states the legal limit is 0.08%
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you choose this answer for 0.0504?
Box 1- 0.08
Box 2- 0.1
Box 3- 0.076
Box 4- 0.0504
\
Why did you choose this answer for 0.0504?
This answer is precise since it measures to 4 decimal places but it is not accurate since it is not close to the true value
17
New cards
What is the difference in 100, 100. and 100.0
The difference is the number of sig figures and that each measurement were made to different degrees of precision
18
New cards
How many sig figures in 0.002304
4
19
New cards
How many sig figs in 0.3450
4
20
New cards
How many sig figures in 1.0230560
8
21
New cards
What is the exact number example?
There are 14 students in this class
22
New cards
What is the number before the multiplication sign called? (1.00 x 10^23)
Mantissa
23
New cards
What is the number after the multiplication sign called? (1.00 x 10^23)
exponent
24
New cards
How many sig figs in 298.0?
4
25
New cards
Convert 298.0 to scientific notation
2\.980 x 10^2
26
New cards
How many sig figs in 0.045
2
27
New cards
Convert 0.045 to scientific notation?
4\.5 x 10^-2
28
New cards
How many sig figs in 3100
2
29
New cards
Convert 3100 to scientific notation
3\.1 x 10^3
30
New cards
How many sig figs in 0.0000001
1
31
New cards
How many sig figs in 1,035,000
4
32
New cards
Convert 1,035,000 to scientific notation
1\.035 x 10^6
33
New cards
What unit would you use to find the mass of a chemical?
Kilograms
34
New cards
What unit would you use to find the volume of a solution?
liters
35
New cards
How many grams are in 1 kilogram?
1000 grams
36
New cards
How many grams are in 2.56 kg?
2560 g
37
New cards
How any kilograms are in 3456 grams
3\.456 kg
38
New cards
How many miligrams are in 1 gram
1000 mg
39
New cards
How many miligrams are in 0.456 g
456 mg
40
New cards
How many grams are in 898 mg?
0\.898
41
New cards
If the mass of an average grape is 1.6 g and someone buys 484 g of grapes (less stems), how many grapes did they buy?
484 g ÷ 1.6 g/grape = 302.5 grapes
**Assuming all the grapes are in fact whole, how can you account for the calculated “half” of a grape?**
484 g ÷ 1.6 g/grape = 302.5 grapes
**Assuming all the grapes are in fact whole, how can you account for the calculated “half” of a grape?**
All of the grapes do not weigh exactly 1.6 g. This results in the calculation of half of a grape.
42
New cards
\n **How would you set up the problem to determine how many of the screws used above were contained in a 50 pound box?**
(50 pounds) x (screws/pound) = screws
43
New cards
When building a deck, many people use screws to hold down the decking. If you decided to use 12 screws per plank and had 100 planks, that would be 1200 screws. Screws are sold by the pound in most hardware stores. If you used screws that are 150 screws per pound, how many pounds of screws do you need to complete the job?
\
\
**If you were unsure how to do this problem, what should you look at to help you find the solution?**
\
\
**If you were unsure how to do this problem, what should you look at to help you find the solution?**
You should arrange the problem to where the units cancel out, and you are left with the units that you want.
44
New cards
When building a deck, many people use screws to hold down the decking. If you decided to use 12 screws per plank and had 100 planks, that would be 1200 screws. Screws are sold by the pound in most hardware stores. If you used screws that are 150 screws per pound, how many pounds of screws do you need to complete the job?
\
**How many of the screws above are contained in a 50 pound box?**
\
**How many of the screws above are contained in a 50 pound box?**
7500 screws
\
(50 pounds) x ( 150 screws/ pound) = 7500 screws.
\
(50 pounds) x ( 150 screws/ pound) = 7500 screws.
45
New cards
What is the mass of two C atoms?
24\.02 amu
\
The mass of two C (carbon) atoms is 24.02. 12.01 amu + 12.01 amu = 24.02 amu. When referring to the mass of the elements on the periodic table, we use the units of amu and not grams.
\
The mass of two C (carbon) atoms is 24.02. 12.01 amu + 12.01 amu = 24.02 amu. When referring to the mass of the elements on the periodic table, we use the units of amu and not grams.
46
New cards
What is the mass of six H atoms?
6\.06 amu
\
6 H atoms have a mass of 6.06 amu. 6 \* 1.01 amu = 6.06 amu
\
6 H atoms have a mass of 6.06 amu. 6 \* 1.01 amu = 6.06 amu
47
New cards
What is the mass of one O atom?
16\.00 amu
48
New cards
What is the combined mass of two C atoms, six H atoms, and one O atom?
46\.08 amu
\
The combined mass of 2 C atoms, 6 H atoms, and 1 O atom is 46.08 because 2(12.01 amu) + 6(1.01 amu) + 16.00 amu = 46.08 amu
\
The combined mass of 2 C atoms, 6 H atoms, and 1 O atom is 46.08 because 2(12.01 amu) + 6(1.01 amu) + 16.00 amu = 46.08 amu
49
New cards
What is the mass of a molecule with the formula C3H9N?
59\.13 amu
50
New cards
What is mass of one dozen molecules of C3H9N?
709\.56 amu
\
12 \* 59.13
\
12 \* 59.13
51
New cards
How many eggs are in 2 dozen?
24
52
New cards
How many stars (6) are in a dozen?
0\.5
53
New cards
How many molecules are in 2 moles of molecules?
1\.204 x 10^24 molecules
\
There are 1.204 x 10^24 molecules in 2 moles of molecules because 2 \* 6.022 x 10^23 = 1.204 x 10^24.
\
There are 1.204 x 10^24 molecules in 2 moles of molecules because 2 \* 6.022 x 10^23 = 1.204 x 10^24.
54
New cards
How many moles of atoms are 2.007 x 10^23 atoms?
0\.3332 mol
\
There are 0.3332 moles in 2.0007 x 10^23 atoms because
(2.007 x 10^23 atoms) ÷ (6.022 x 10^23 moles/atom) = 0.3332 moles.
\
There are 0.3332 moles in 2.0007 x 10^23 atoms because
(2.007 x 10^23 atoms) ÷ (6.022 x 10^23 moles/atom) = 0.3332 moles.
55
New cards
What is the average mass for a single atom of Na?
22\.99 amu
56
New cards
What is the average mass for 6.022 x 10^23 atoms of Na?
22\.99 g
57
New cards
What is the average mass of one mole of Na?
22\.99 g
58
New cards
What is the average mass for 0.50000 mole Na atoms?
11\.49 g
\
0\.5 x 22.9
\
0\.5 x 22.9
59
New cards
is it necessary to use the value of Avogadro's number (6.022 x 10^23) to determine the mass of 1.0000 mole or 0.50000 mole of Na atoms? Why or why not?
No, you do not need Avogadro's number because the periodic table tells the mass in grams of one mole of Na. (Moles) x (g/mol) = grams
60
New cards
What is the mass of 2 mol C?
24\.02 g
\
The mass of 2 mol of C is 24.02 g because 2 mol C x (12.01 g/mol) = 24.02 g.
2 x 12.01 g= 24.02 g
\
The mass of 2 mol of C is 24.02 g because 2 mol C x (12.01 g/mol) = 24.02 g.
2 x 12.01 g= 24.02 g
61
New cards
What is the mass of 6 mol H?
6\.06 g
62
New cards
what is the mass of 1 mol of O?
16\.00
63
New cards
What is the combined mass of 2 mol C, 6 mol H, and 1 mol O?
46\.08 g
64
New cards
What is the mass of one mole of molecules with the formula C3H9N?
59\.13 g
65
New cards
What is the mass of 3.3 moles of molecules of C3H9N?
195\.1 g
66
New cards
**How many eggs are contained in the following?**
\
0\.25 dozen eggs
\
0\.25 dozen eggs
3 eggs
\
0\.25 x 12
\
0\.25 x 12
67
New cards
**How many eggs are contained in the following?**
\
0\.85 moles of eggs
\
0\.85 moles of eggs
5\.119 x 10^23
\
0\.85 moles of eggs x (6.022 x 10^23 eggs/mol) = 5.119 x 10^23 eggs
\
0\.85 moles of eggs x (6.022 x 10^23 eggs/mol) = 5.119 x 10^23 eggs
68
New cards
**How many carbon atoms are contained in the following?**
\
3\.48 moles of carbon atoms
\
3\.48 moles of carbon atoms
2\.10 x 10^24
\
3\.48 moles of C x (6.022 x 10^23 atoms/mol) = 2.10 x 10^24
\
3\.48 moles of C x (6.022 x 10^23 atoms/mol) = 2.10 x 10^24
69
New cards
**How many carbon atoms are contained in the following?**
\
0\.035 moles of carbon atoms
\
0\.035 moles of carbon atoms
2\.1 x 10^22
\
There are 2.1 x 10^22 carbon atoms in 0.035 moles of carbon atoms.
\
There are 2.1 x 10^22 carbon atoms in 0.035 moles of carbon atoms.
70
New cards
Would you expect 8.8 x 10^23 carbon atoms to be greater than, less than or equal to 1 mole?
Greater then.
71
New cards
How many moles of carbon atoms are found in 8.8 x 10^23 carbon atoms?
1\.5 moles.
\
8\.8 x 10^23 C atoms x ( 1 mol/6.022 x 10^23 atoms) = 1.5
\
8\.8 x 10^23 C atoms x ( 1 mol/6.022 x 10^23 atoms) = 1.5
72
New cards
Would you expect 6.24 x 10^22 carbon atoms to be greater than, less than or equal to 1 mole?
Less
73
New cards
How many moles of carbon atoms are found in 6.24 x 10^22 carbon atoms?
0\.104 moles
\
6\.24 x 10^22 x (1 mol/6.022 x 10^23 atoms)=0.104
\
6\.24 x 10^22 x (1 mol/6.022 x 10^23 atoms)=0.104
74
New cards
**If an average egg weighs 50 g, how much does each of the following weigh?**
\
1 dozen eggs
\
1 dozen eggs
600 g
75
New cards
**If an average egg weighs 50 g, how much does each of the following weigh?**
\
1 mole eggs
\
1 mole eggs
3\.01 x 10^25 g
\
50 g x (6.022 x 10^23 moles/g) = 3.01 x10^25 g
\
50 g x (6.022 x 10^23 moles/g) = 3.01 x10^25 g
76
New cards
**If an average atom of carbon weighs 1.9945 x 10^-23 g, how much does each of the following weigh?**
\
1 dozen carbon atom
\
1 dozen carbon atom
2\.3934 x 10^-22 g
\
12 C atoms x (1.9945 x 10^-23 g/atom) = 2.3934 x 10^-22 g
\
12 C atoms x (1.9945 x 10^-23 g/atom) = 2.3934 x 10^-22 g
77
New cards
What is the mass of 1.35 mole O2 molecules?
43\.2 g
\
1\.35 mole x (32.0 g/ mol) = 43.2 g
\*\*O2 = 2 x 16 = 32
\
1\.35 mole x (32.0 g/ mol) = 43.2 g
\*\*O2 = 2 x 16 = 32
78
New cards
Is the reaction N2(g) + H2(g) → NH3(g) balanced?
No
79
New cards
For the balanced reaction CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g), **how many molecules of CH4 are required to make 2 molecules of H2O?**
1 molecule
80
New cards
Based on that ratio determined in Q4, **how many moles of O2 are required to completely consume 9 moles of CH4? (1:2 ratio)**
18 moles of O2
81
New cards
If 4 moles of CH4 were used in the reaction CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g), **what is the maximum number of moles of CO2 you could expect the reaction to produce?**
4 moles. (1:1 ratio)
82
New cards
What is the maximum number of moles of H2O you could expect the reaction to produce if 4 moles of CH4 were used?
8 moles of H2O
83
New cards
If only 0.5 moles of O2 were used in the reaction CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g), **what is the maximum number of moles of CO2 you could expect the reaction to produce?**
0\.25 moles.
84
New cards
How many moles are contained in 24 g CH4?
1\.5 moles
\
24 g x (1 mol/ 16.04 g) = 1.5 moles
\
24 g x (1 mol/ 16.04 g) = 1.5 moles
85
New cards
How many moles of CO2 could be produced if 24g of CH4 were used in the reaction CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)?
1\.5 moles of CO2
\
Found the moles of CH4 and its same number since 1:1 ratio.
\
Found the moles of CH4 and its same number since 1:1 ratio.
86
New cards
What type of mixture is antifreeze in water?
Homo mixture
87
New cards
What type of mixture is brass (copper and zinc)
homo mixture
88
New cards
what mixture is dry air
homo mixture
89
New cards
what mixture is black coffee
homo mixture
90
New cards
what mixture is real bannana pudding
hetero mixture
91
New cards
what type of mixture is ice tea
hetero mixture
92
New cards
What are all the possible solutes in Dr. Pepper
Carbon dioxide, sugar, flavoring
93
New cards
Is 1 teapsoon of salt in water statured or unsaturated?
Unsaturated
94
New cards
Is 1 pound of salt in water statured or unsaturated?
Statured
95
New cards
Is 1 teaspoon of sand in water statured or unsaturated?
Statured
96
New cards
Is 1 teaspoon of baking soda in water statured or unsaturated?
Unsaturated
97
New cards
Is 1 teaspoon of acetone in water statured or unsaturated?
Unsaturated
98
New cards
What is the solvent in Dr. Pepper
Water
99
New cards
Which would be more soluble at 10C, a gas or a solid?
Gas
100
New cards
Which would be more soluble at 90C, a gas or a solid?
Solid