Aromatic Compounds And Amines
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
What is the molecular formula of a benzene ring on it’s own?
C6H6
What is the formula of a benzene ring as a side chain?
C6H5
How was benzene’s molecular formula found?
Discovered in 1852 by Michael Faraday
Scientists didn’t know about it’s cyclic structure
They determined it’s empirical formula to be CH and it’s Mr to be 78
So the molecular formula must be C6H6
What was Kekule’s theory of what benzene was called?
Resonance Hybrid Theory
What was the suggested structure of benzene in resonance hybrid theory?
The IUPAC name is cyclohexa-1,3,5-triene
The theory suggested the C=C changed position and resonates between two structures
What were three problems with Kekule’s theory of the benzene ring?
Did not decolourise bromine water
The structure should undergo electrophilic addition similar to other unsaturated compounds
Therefore benzene has a lower chemical reactivity than what would be predicted by Kekule
The enthalpy of hydrogenation does not agree with the structure
The enthalpy of hydrogenation for cyclohexene is -120KJmol-1 so naturally to find cyclohexa-1,3,5-triene we would multiply this by 3
The actual enthalpy of hydrogenation was -208KJmol-1 meaning the structure is more stable than Kekule’s structure as it releases less energy
X-ray diffraction by Kathleen Londsdale at Leeds uni proved the structure incorrect
All the bond lengths were equal- if there were 3 C-C bonds and 3 C=C bonds it would have had longer C-C bonds and shorter C=C bonds
X-ray studies showed the bond length to be 0.140nm between single and double bond length
What is the true model of the benzene ring called?
The delocalised ring model
What is the delocalised ring model?
It shows 6 carbons in a cyclic compound with only one hydrogen attached to each one
There is a overlap of sigma bonds between carbons and an overlap of pi bonds above and below each carbon’s place
The pi cloud is made up of 6 delocalised electrons and is shown as a circle when drawing benzene
The electrons can move between pi bonds
What is the shape of benzene?
Each carbon bonded three atoms
Planar
Hexagonal
Why does benzene have a stable structure?
Each carbon has 3 bonds and 1 delocalised electron
The delocalised electrons in the pi bonds are not fixed in place but are delocalised around the benzene ring, making it more stable
How do we name aromatics?
Benzenes are above alkenes, alkanes, nitro compounds and halogens in naming priority
Examples: Methyl benzene or chloro benzene
In compounds where higher priority groups are present, benzene has the prefix ‘phenyl’
Example: Phenyl ethanoic acid
What is the only time we can get the ketone “ethanone”?
Only if a benzene ring is attached as it is classed as a side chain
What is benzene classed as in compounds where it is not the highest priority group?
A side chain
What species readily attack benzene rings and why?
Electrophiles
The 6 delocalised electrons creates a region of high electron density
Why can benzene rings not undergo electrophilic addition?
It would disrupt the benzene ring
What happens in electrophilic substitution?
Electrons from the delocalised ring move towards the electrophile
The arrow must come from the ring itself to the electrophile
The ring then becomes temporarily disrupted
2 electrons are used to form a dative covalent bond with the electrophile
The benzene ring must be shown with the opening facing the place where the electrophile was added and with a positive charge
The C-H bond breaks and the electrons move back into the benzene ring
The arrow must go from the bond to the middle of the disrupted ring
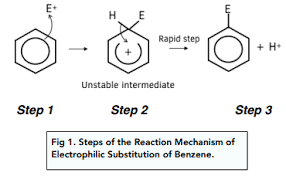
Why are extreme conditions needed for electrophilic substitution to occur?
Because benzene is fairly unreactive- it is stable
What type of mechanism is nitration and what are the conditions and reagents for the reaction?
Electrophilic substitution
Concentrated nitric acid and concentrated sulfuric acid at 50oC
You have to put concentrated in front of both acids cause AQA are top tier ragebaiters
Requires the nitronium ion (NO2+)
What is nitration and what is needed to generate the electrophile for nitration?
Nitration is the addition of a NO2+ ion
Sulfuric acid acts as a catalyst and is needed to generate the nucleophile
What is the equation for the formation of NO2+?
HNO3 + H2SO4 → NO2+ + HSO4- + H2O
What do you have to remember when showing NO2+ as a nucleophile?
The positive charge always goes on the nitrogen
What are some uses of aromatic compounds?
Drugs
Dyes
Explosives
Plastics
What are some uses of nitrobenzene?
Drugs and manufacture of dyes
How can we show that in the nitration of benzene, sulfuric acid is a catalyst?
H+ is produced in the nitration of benzene
HSO4- is also produced in the reaction of concentrated sulfuric acid with concentrated nitric acid to form NO2+
So:
HSO4- + H+ → H2SO4
What is the overall equation for the nitration of benzene?
C6H6 + HNO3 → C6H5NO2 + H2O
H2SO4 and 50oC on the arrow
Why is nitration carried out at 50 degrees celcius?
Otherwise multiple substitution occurs
What can happen in the nitration of methylbenzene above 50oC and why?
Multiple substitution occurs which can form a compound called trinitrotoluene, also known as TNT
TNT go boom so its not very safe
Methyl groups are electron releasing making the benzene ring more electron dense, so electrophiles are even more attracted to it
What is the mechanism for Friedel-Crafts Acylation?
Electrophilic substitution
What happens in Friedel-Crafts Acylation?
Benzene can react with acyl chlorides in the presence of an aluminium chloride catalyst (also known as a halogen carrier catalyst) to form an aromatic ketone
What is the equation to form the electrophile for Friedel-Crafts Acylation and what conditions must this occur in?
CH3COCl + AlCl3 → CH3C+O + AlCl4-
This must take place in anhydrous conditions
How can we show AlCl3 as a catalyst?
The acylation of benzene produced H+
The equation to form the electrophile (CH3C+O) produces AlCl4-
So:
AlCl4- + H+ → AlCl3 + HCl
What is the overall equation for the Friedel-Crafts Acylation of benzene?
C6H6 + CH3COCl → C6H5COCH3 + HCl
AlCl3 on the arrow
What are amines?
Nitrogen containing compounds derived from ammonia
On or more of the hydrogen atoms has been replaced by an alkyl group
What is the difference between a primary, secondary and tertiary amine?
Primary- 1 alkyl group replacing one hydrogen
Secondary- 2 alkyl group replacing two hydrogens
Tertiary- 3 alkyl groups replacing 3 all hydrogens
Suffix and prefix for amines?
Suffix- -amine
Prefix- amino-
For N substituted amines, what is the N used for?
It is used to show whatever comes after is directly attached to the nitrogen
What two things can amines act as and what is their meaning?
Nucleophile- electron pair donor
Base- H+ acceptor
How can amines be shown to be basic?
They can form dative covalent bonds with hydrogen ions to form alkyl ammonium ions
Write an equation to show the base strength of an amine
NH2 + H+ ⇌ RNH3
What does the base strength of an amine depend on?
The availability of the lone pair of electrons on the nitrogen atom
The amine will be a stronger base if the lone pair of electrons are more available to accept a proton
Why does methylamine have a greater basicity than phenylamine?
The lone pair of electrons on N is more readily available to accepted H+ because the methyl group is releasing electrons towards it
On phenylamine, the lone pair of electrons on N becomes partially delocalised in the benzene ring, making the lone pair on N less readily available to accept H+
Why is phenylmethanamine more basic than phenylamine?
The benzene ring is too far removed from the nitrogen to cause the lone pair of electrons on N to become partially delocalised in the benzene ring
What can happen in the multiple nucleophilic substitution of bromopropane with ammonia?
The ammonia reacts with bromopropane to form propan-1-amine
The nitrogen however has a lone pair on it, so the product can acts as a nucleophile and attack another delta positive carbon on a bromopropane molecule
This repeats itself, forming dipropylamine and then tripropylamine which both still have a lone pair of electrons on them
This can undergo nucleophilic substitution one more time to form a tetrapropyl ammonium ion which is a quaternary ammonium ion where the nitrogen has a positive charge
The bromine may form an ionic bond with this compound to form a quaternary ammonium salt
To produce only primary amines from nucleophilic substitution with halogenalkanes, what do we need?
An excess of ammonia
(This can still result in 2o, 3o and 4o impurities)
If we want 2o, 3o or 4o amines from nucleophilic substitution with halogenalkanes, what do we need?
An excess of the halogenalkane
What is a use of quaternary ammonium salts and how does it work?
Cationic surfactants in fabric conditioners and hair products
They coat the surface of fabric/hair with positive charges and reduce the static due to negatively charged electrons
What is an example of a quaternary ammonium ion in the body?
Acetylcholine
Because it is positively charged, it can carry the electrical charge from one synapse to another
Why is nucleophilic substitution not the best way to prepare amines?
Produces secondary, tertiary and quaternary amines as impurities
What is a better method of producing amines?
Reduction of nitriles
Using nitrogen in the presence of a nickel catalyst of LiAlH4 in dry ether (usually represented by [H])
What are some different names for the preparation of amines using hydrogen in the presence of a nickel catalyst or LiAlH4?
Reduction
Hydrogenation
Catalytic addition
What is the large scale process for the preparation of amines, what are the conditions and what is the equation for this reaction?
Preparation using hydrogen in the presence of a nickel catalyst
RC≡N + 2H2 → RNH2
High temperature and pressure required
The 4 hydrogens are added onto the carbon and the nitrogen
What is the small scale process for the preparation of amines, what are the conditions and what is the equation for this reaction?
Preparation of amines using hydrogen in the presence of LiAlH4 in dry ether
RC≡N + 4[H] → RNH2
The 4 hydrogens are added onto the carbon and the nitrogen
What is an aliphatic amine?
A compound where nitrogen is directly bonded to one more more saturated alkyl groups
Basically a non-aromatic amine
How can aromatic amines be produced?
From the reduction of nitro compounds
Write the equation for the reduction of nitro compounds to form an aromatic amine, also state the conditions.
C6H5NO2 + 6[H] → C6H5NH2 + 2H2O
This uses a tin catalyst with concentrated sulfuric acid (i think ill ask miss)
This acts as a reducing agent
How can we distinguish between methylamine and ammonia dissolved in water?
Use a pH probe
pH of ammonia will be lower than methylamine
Why can phenylamine not be prepared from bromobenzene by nucleophilic substitution?
Because the benzene ring/delocalise electrons in the pi cloud repel electrons on the nucleophile
Arrange these bonds in terms of increasing bond length:
C-C in a cyclic compound
C=C in a cyclic compound
C-C in a benzene ring
C=C in cyclic
C-C in benzene
C-C in cyclic
Why is the reaction of aldehydes with HCN faster than ketones?
The alkyl groups in ketones hinder attack because they are releasing electrons towards the delta positive carbon (positive inductive effect)
Using your knowledge of the bonding in benzene, why does cyclohexa-1,4-diene have a greater enthalpy of hydrogenation that cyclohexa-1,3-diene?
The P orbitals in 1,3 are close enough that the pi electrons can delocalise across the molecule
The p orbitals in 1,4 are 2 carbons apart so the p orbitals cannot overlap with each other, preventing the delocalisation of pi electrons across the molecule