C2.4 Organic Compounds (Basics)
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
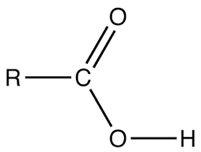
carboxylic acid
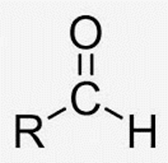
Aldehyde
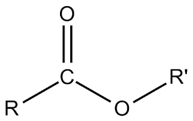
Ester
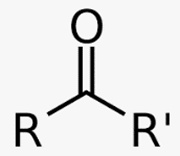
Ketone
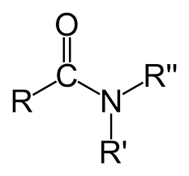
Amide
General family name
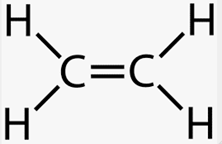
Alkene
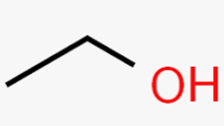
Alcohol
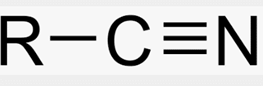
Nitrile
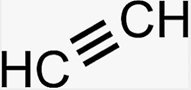
General family term
Alkyne
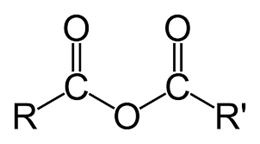
Acid anhydride
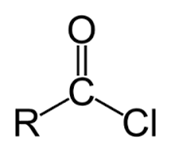
Acid chloride
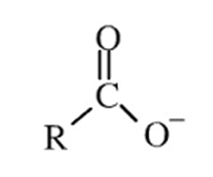
Carboxylate ion
Skeletal formula
A formula that shows just the carbon backbone and any functional group. |
Displayed formula
a formula showing all bonds and all atoms
Structural formula
A formula showing groups of atoms but few/no bonds
General formula
an algebraic formula applied to homologous series
Carboxylic acid suffix
oic acid
Aldehyde suffix
al
Ketone suffix
one
Ester suffix
oate
Amide suffix
amide
Alkene suffix
ene
Alkane suffix
ane
Alcohol suffix
ol
Nitrile suffix
nitrile
Alkyne suffix
yne
Amine suffix
amine
Amine prefix
amino
Alcohol prefix
hydroxy
Saturated carbon chain prefix
yl
Addition reaction
two or more substances reacting to form one
Substitution reaction
Part of one molecule swapping with another part of another molecule
Elmination
A reaction where a small molecule is released
Hydrolysis
using water to cause the breaking of a bond(s)
Oxidation
addition of oxygen or removal of hydrogen
Reduction
Removal of oxygen or addition of hydrogen
Electrophile
lone pair acceptor
Nucleophile
lone pair donor
Radical
a highly excited species with an unpaired electron
Homolytic fission
bond breaking where the covalent bond splits equally forming radicals
Heterolytic fission
where the covalent bond breaks unevenly forming ions
Monomer
a single unit that joins together to make a polymer
Polymer
a long repeating chain of many monomers
Structural isomer
same molecular formula but different arrangement of atoms
Increasing chain length
increases melting point and boiling point of hydrocarbons
Hydrocarbon
a compound made only of carbon and hydrogen