Chemistry Unit 2
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Indications of a Chemical Reaction
evolution as heat & or light
production of a gas
Formation of a precipitate (solid produced as a result of a CR in solution that separated from a solution)
Color change
Sound
Law of conservation of Mass
atoms can neither be created nor destroyed
Synthesis
combine 2 substances into 1
Decomposition
1 thing breaks into more substances
Single Replacement
an atom trades places with 1 part of a molecule in order to change they have to have the same charge
Double replacement
2 compounds switch places Balance :
Switch pos with neg first
Change to fit charges
Balance regularly
Combustion
Carbon Compound + O2 —> CO2+H2O
CxHy+O2→CO2+H2O
Diatomic Molecules
H,N,O,Br,I,CL,F (Brincl-Hof) or (I Have No Class On Friday Bro)
Collision Theory
to have a reaction particles must collide & they have to be energetic + be in the correct orientation
Activation Energy
Minimum amount of extra energy required to for the reaction to happen
Reaction Rate
Change in the concentration of reactants as time goes by
Chemical Equilibrium
state where forward reaction = backwards reaction
Le Chatelier’s
stress effects equilibrium) incl Concentration, Pressure & Temp
Concentration A+B=C+D
add more→making more products →products →right
take away →use products to replace missing →making more reactants →left
Pressure based on mol’s on either side
Inc pressure (dec vol) →less space →go to less GAS mol’s to fit in smaller space
dec pressure (inc vol) →have more space→go to more GAS mol’s to fill up the space
Temperature 2 types Endo Exo
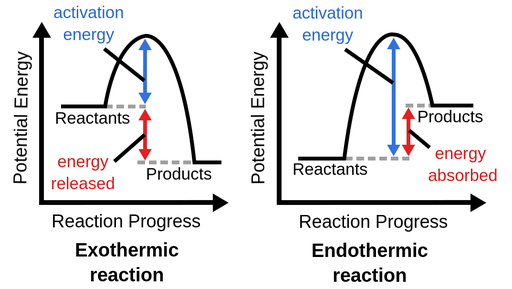
Temp under Chat Endothermic Heat+A=B
inc tmep is adding heat so shift right (bc it wants to cool down)
dec temp is removing heat so shift left (bc it wants to warm up)
products being lower (means you RELEASE energy) EXO
Temp under Chat Exothermic A=B+Heat
inc temp : left to get cooler
dec temp : right to get warmer
products being higher (means you ABSORB energy) ENDO
Which states of matter do not affect equilibrium?
pure solids & liquids
Three conditions for a reaction to be completed? (no =)
solid forms, gas forms and escapes, Ionic bonds form