14 - hydrocarbons
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
alkanes
Alkanes are saturated hydrocarbons that can be produced by the addition reaction of hydrogen to an alkene or by cracking of longer alkane chains
their general formula is : CnH2n+2
naming alkanes - rules (applicable to other organic compounds too)
Select the longest carbon chain that contains the highest number of substituents.
Number the carbon chain in order to give the substituents the lowest possible numbers
Place the substituents according to the alphabetical order as prefixes indicating their respective
positions.
If more than one substituent of a given type is present, indicate their number by the numerical
terms , di ( two ) , tri ( three ) ,tetra ( four ) and so on.
Numbers and names are separated by a hyphen ( – ) and numbers are separated by a
comma.
End the name of the alkane with the suffix , ane.
cycloalkanes
Cycloalkanes are a group of saturated hydrocarbons that share the same general formula CnH2n with alkenes.
Production of alkanes from addition reactions
Alkenes are unsaturated organic molecules containing at least one C=C double bond
Alkenes can be heated with hydrogen gas and a Pt/Ni catalyst
The Pt/Ni catalyst is finely divided to increase its surface area
This increases the overall rate of reaction
This is an addition reaction which forms an alkane from an alkene, for example:
butene + hydrogen →(Pt H2) butane
Alkanes are formed from hydrogenation. - The addition reaction of alkenes with hydrogen is called hydrogenation

where is hydrogenation used in the industry
Margarine Production from Vegetable Oil
Vegetable oils are typically derived from plant sources and consist mainly of unsaturated fatty acids—hydrocarbon chains that contain one or more C=C double bonds.
These double bonds introduce kinks in the chains, which prevent the molecules from packing closely together. As a result, vegetable oils are liquids at room temperature.
In the production of margarine, these oils undergo partial hydrogenation:
Hydrogen gas (H₂) is bubbled through the oil.
A nickel catalyst is used.
The reaction is carried out at around 150–200°C under high pressure.
During this process:
Some of the C=C double bonds are converted into C–C single bonds as hydrogen atoms are added across the double bonds.
This makes the hydrocarbon chains more saturated and straighter.
As the chains become straighter:
They can pack more tightly together.
Intermolecular forces (Van der Waals) between the molecules become stronger.
This results in a higher melting point.
Therefore, the product (margarine) becomes a semi-solid at room temperature, making it spreadable, unlike the original liquid vegetable oil.
⚠ Note: Complete hydrogenation would make the product too hard, so only partial hydrogenation is used.
In older methods, partial hydrogenation sometimes produced trans fats (trans isomers), which have negative health effects. Modern manufacturing aims to minimize or eliminate trans fat formation.c
cracking definition and why we crack hydrocarbons
Cracking is a process where large hydrocarbon molecules (usually alkanes) are broken down into smaller, more useful hydrocarbons — like shorter-chain alkanes and alkenes.
Long-chain alkanes from crude oil are less useful (too viscous, hard to ignite).
We crack them to make short-chain alkanes (fuels) and alkenes (feedstock for polymers and chemicals).
Cracking increases the supply of shorter-chain hydrocarbons, which are in higher demand.
Cracking also increases the supply of alkenes, which do not occur in crude oil naturally but are very important for industrial chemistry
production of alkanes from cracking
In cracking large, less useful hydrocarbon molecules found in crude oil are broken down into smaller, more useful molecules - primarily smaller alkanes and alkenes.
The large hydrocarbon molecules are fed into a steel chamber, heated to a high temperature and then passed over an aluminium oxide (Al2O3) catalyst
The chamber does not contain any oxygen to prevent combustion of the hydrocarbon to water and carbon dioxide
When a large hydrocarbon is cracked, a smaller alkane and alkene molecules are formed
E.g. octane and ethene from decane

cracking in detail
The large hydrocarbon is vaporised (turned into gas) by heating to about 450–750°C.
It is then passed over a hot catalyst, typically aluminium oxide (Al₂O₃) or silicon dioxide (SiO₂) — often supported on a ceramic base.
The reaction chamber is kept in anaerobic conditions (no oxygen) to prevent combustion (oxidation to CO₂ and H₂O).
Bonds in the large alkane molecules break heterolytically or homolytically, depending on conditions, producing smaller alkanes and alkenes.
⚗ Types of Cracking (mention briefly):
Catalytic cracking:
Uses a catalyst (like Al₂O₃), lower temperature (~450°C), and moderate pressure.
Produces branched alkanes, aromatic hydrocarbons, and alkenes.
Used in the petroleum industry for making motor fuel.Thermal cracking:
Uses higher temperatures (up to 900°C) and high pressure, no catalyst.
Produces mainly alkenes, which are used in polymer manufacture.

what type of reactions are hydrogenation and cracking (exo or end)
hydrogenation is an exothermic reaction and cracking is an endothermic reaction
reactions of alkanes
Alkanes are generally unreactive compounds. The very small difference in electronegativity between carbon and hydrogen makes the C – H bonds non polar and alkane molecules non polar overall. Alkanes are not attacked by electrophiles or nucleophiles.
The reactions of alkanes are limited only to combustion on a large scale for their use as fuels and free radical substitution to form more reactive halogenalkanes.
alkanes in complete combustion
When alkanes are burnt in excess (plenty of) oxygen, complete combustion will take place and all carbon and hydrogen will be oxidised to carbon dioxide and water respectively
For example, the complete combustion of octane to carbon dioxide and water

incomplete combustion of alkanes
When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised
Some carbon is only partially oxidised to form carbon monoxide
For example, the incomplete combustion of octane to form carbon monoxide

carbon monoxide gas
Carbon monoxide is a toxic gas as it will bind to haemoglobin in blood which can then no longer bind to oxygen
As no oxygen can be transported around the body, victims will feel dizzy, lose consciousness and if not removed from the carbon monoxide, they can die
Carbon monoxide is extra dangerous as it is odourless (it doesn’t smell) and will not be noticed
Incomplete combustion often takes place inside a car engine due to a limited amount of oxygen present
free-radical substitution of alkanes
Alkanes can undergo free-radical substitution in which a hydrogen atom gets substituted by a halogen (chlorine/bromine)
Since alkanes are very unreactive, ultraviolet light (sunlight) is needed for this substitution reaction to occur
The free-radical substitution reaction consists of three steps:
In the (chain) initiation step, the halogen bond (Cl-Cl or Br-Br) is broken by UV energy to form two radicals through homolytic fission
These radicals create further radicals in a chain type reaction called the (chain) propagation step (A chlorine free radical attacks a molecule of methane and continues to propagate the chain reaction. One C – H bond at a time breaks homolytically until CH4 turns in to CCl4. Each step produces a free radical.)
The reaction is terminated when two radicals collide with each other in a (chain) termination step
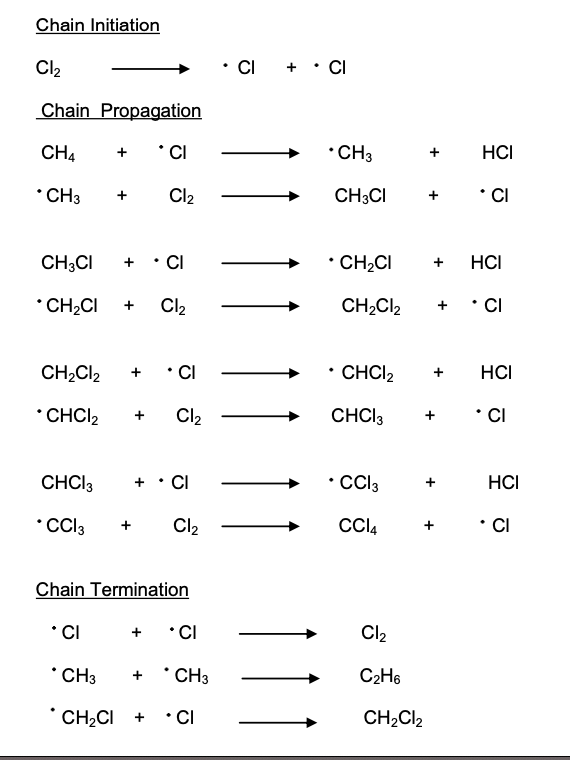
free-radical substitution of alkanes - steps
-
reacting an alkane with bromine
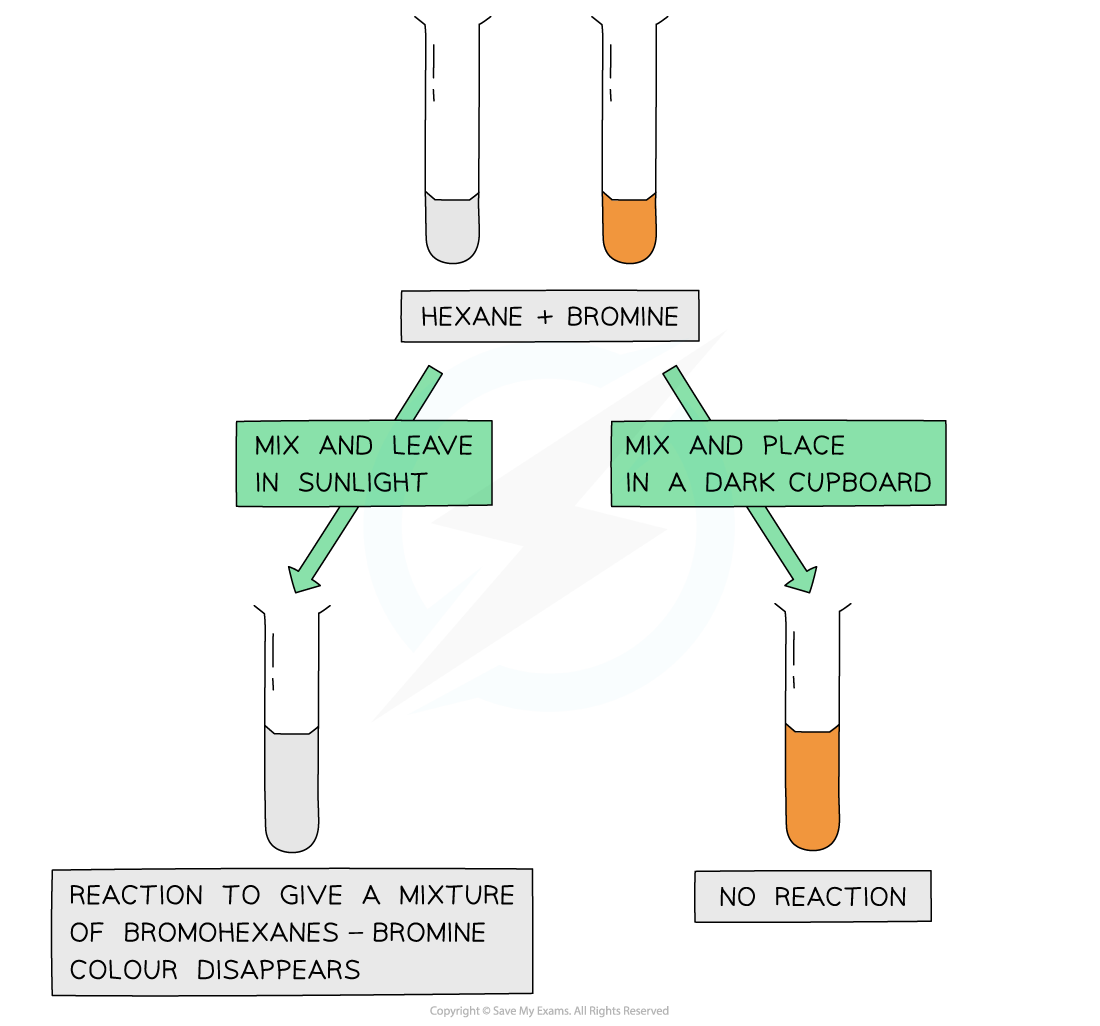
The fact that the bromine colour has disappeared only when mixed with an alkane and placed in sunlight suggests that the ultraviolet light is essential for the free radical substitution reaction to take place
initiation step in free-radical substitution of alkanes
In the initiation step, the halogen bond (Cl-Cl or Br-Br) is broken by UV energy to form two radicals
The covalent Cl-Cl bond is broken by energy from the UV light
Each atom takes one electron from the covalent bond
This produces two radicals in a homolytic fission reaction
Cl–Cl 2Cl•
propagation step in free-radical substitution of alkanes
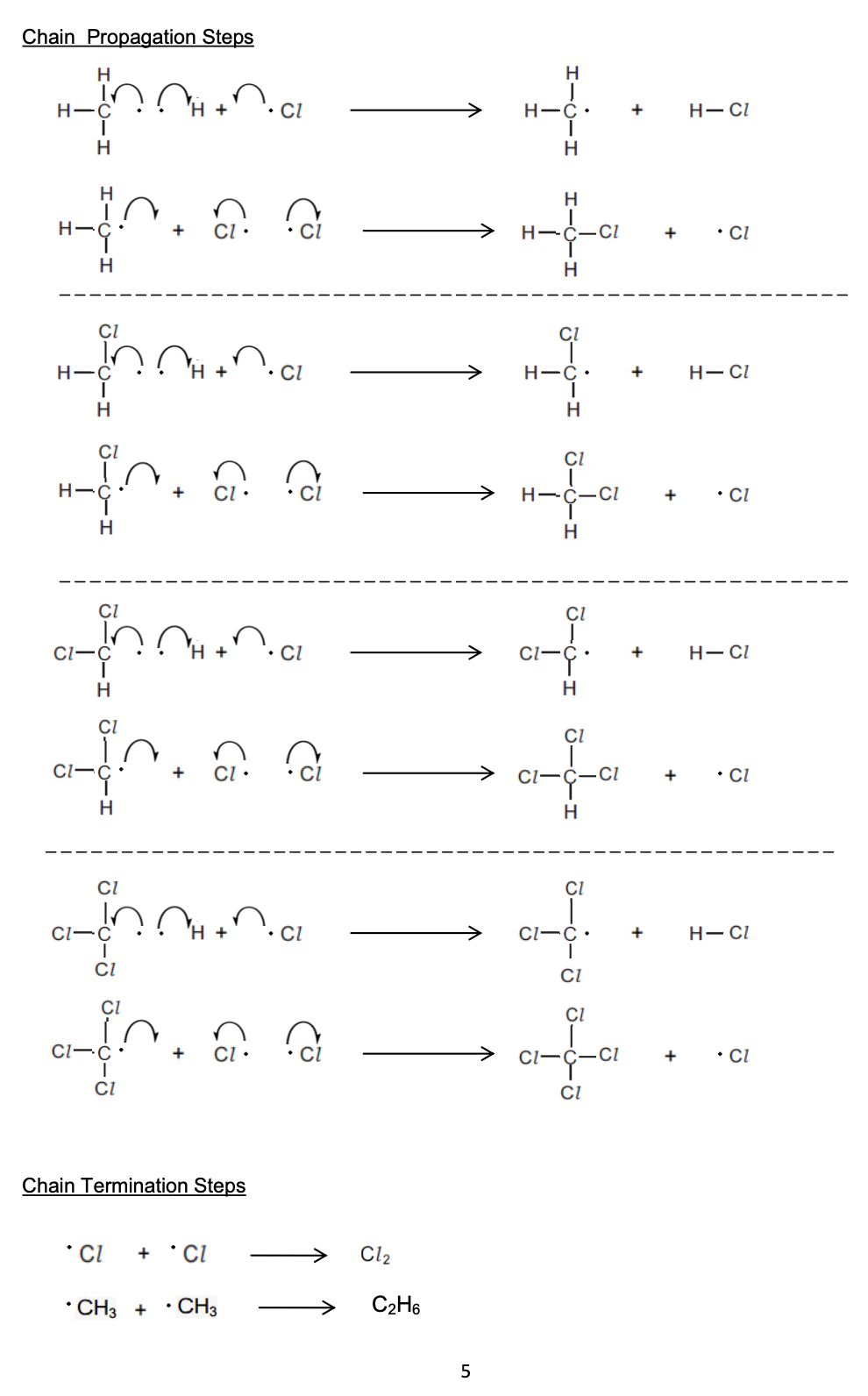
The halogen free radicals are very reactive and will attack the unreactive alkanes
One of the methane C-H bond breaks homolytically to produce an alkyl radical
CH4 + Cl• → •CH3 + HCl
The alkyl radical can attack another chlorine molecule to form a halogenoalkane
This also regenerates the chlorine free radical
•CH3 + Cl2 → CH3Cl + Cl•
The regenerated chlorine free radical can then repeat the cycle
For example, the chlorination of ethane is:
ethane + chlorine radical → ethyl radical + hydrogen chloride
CH3CH3 + Cl• → •CH2CH3 + HCl
ethyl radical + chlorine molecule → chloroethane + regenerated chlorine radical
•CH2CH3 + Cl2 → CH3CH2Cl + Cl•
This reaction is not very suitable for preparing specific halogenoalkanes as a mixture of substitution products is formed
If there is enough halogen present, all the hydrogens in the alkane will eventually get substituted
For example, the chlorination of ethane could continue:
chloroethane + chlorine radical → radical + hydrogen chloride
CH3CH2Cl + Cl• → •CH2CH2Cl + HCl
radical + chlorine molecule → 1,2-dichloroethane + regenerated chlorine radical
•CH2CH2Cl + Cl2 → CH2Cl2 + Cl•
This process can repeat until hexachloroethane, C2Cl6, is formed
last step in free-radical substitution of alkanes
The termination step is when the chain reaction terminates (stops) due to two free radicals reacting together and forming a single unreactive molecule
Multiple products are possible
For example, the single substitution of ethane by chlorine can form:
ethyl radical + chlorine radical → chloroethane
•CH2CH3 + Cl• → CH3CH2Cl
ethyl radical + ethyl radical → butane
•CH2CH3 + •CH2CH3 → CH3CH2CH2CH3
chlorine radical + chlorine radical → chlorine molecule
Cl• + Cl• → Cl2
examiner tips and tricks - If you are asked to give an equation for the termination step of a free radical reaction / mechanism, you should not give the equation reforming the original halogen as this is often marked as "ignore" on mark schemes.
Free radical substitution using bromine instead of chlorine is possible and follows similar initiation, propagation and termination steps
-
further substitution in alkanes
Often, free radical reactions are not very suitable for preparing specific halogenoalkanes as a mixture of substitution products are formed
If there is enough chlorine / bromine present, all the hydrogens in the alkane will eventually get substituted
For example, methane could be substituted to become chloromethane and then further substituted
(Free radical substitution is a very reactive and uncontrolled process.Once it starts, it doesn't just stop at making one product.
Instead, it keeps going and can replace more than one hydrogen atom in the alkane.
So, you don’t just get the product you want — you get a mixture of different halogenoalkanes.)
Single substitution:
CH4 + Cl• → •CH3 + HCl
•CH3 + Cl2 → CH3Cl + Cl•
Second substitution:
CH3Cl + Cl• → •CH2Cl + HCl
•CH2Cl + Cl2 → CH2Cl2 + Cl•
Third substitution:
CH2Cl2 + Cl• → •CHCl2 + HCl
•CHCl2 + Cl2 → CHCl3 + Cl•
Complete substitution:
CHCl3 + Cl• → •CCl3 + HCl
•CCl3 + Cl2 → CCl4 + Cl•
examiner tips and tricks
You could be asked to draw the mechanism for initiation and termination steps for free radical substitution
This mechanism will use half-headed arrows to show the movement of one electron (double-headed arrows show the movement of a pair of electrons)
A half-headed arrow is known as a ‘fish hook’ arrow.
Initiation:

Termination:

The key is the use of the ‘fish hook’ arrow to show the homolytic fission of the bond in initiation and the formation of the bond in termination.
crude oil
Crude oil is a natural, unrefined fossil fuel formed over millions of years from the remains of ancient marine organisms (like plankton) buried under sedimentary rock. Under high pressure and temperature, these remains were chemically transformed into a mixture of hydrocarbons containing alkanes, cycloalkanes and arenes (compounds with a benzene ring)
The crude oil is extracted from the earth in a drilling process and transported to an oil refinery
how is crude oil separated
At the oil refinery, the crude oil is separated into useful fuels by fractional distillation
This is a separating technique in which a wide range of different hydrocarbons are separated into fractions based on their boiling points
Crude oil is heated and partially vaporised.
The vapour enters a fractionating column — hot at the bottom, cooler at the top.
As vapours rise, they cool and condense at different levels depending on their boiling points.
The hydrocarbons are collected as fractions, each containing molecules of similar chain length and boiling range.
main fractions in fractional distillation with their uses
Fraction | Carbon atoms | Use |
|---|---|---|
Refinery gas | C1–C4 | Bottled gas, LPG |
Petrol (Gasoline) | C5–C12 | Car fuel |
Naphtha | C5–C10 | Petrochemicals |
Kerosene | C10–C16 | Jet fuel |
Diesel | C14–C20 | Lorry and bus fuel |
Fuel oil | C20–C70 | Ship/industrial fuel |
Bitumen | C70+ | Road surfacing |
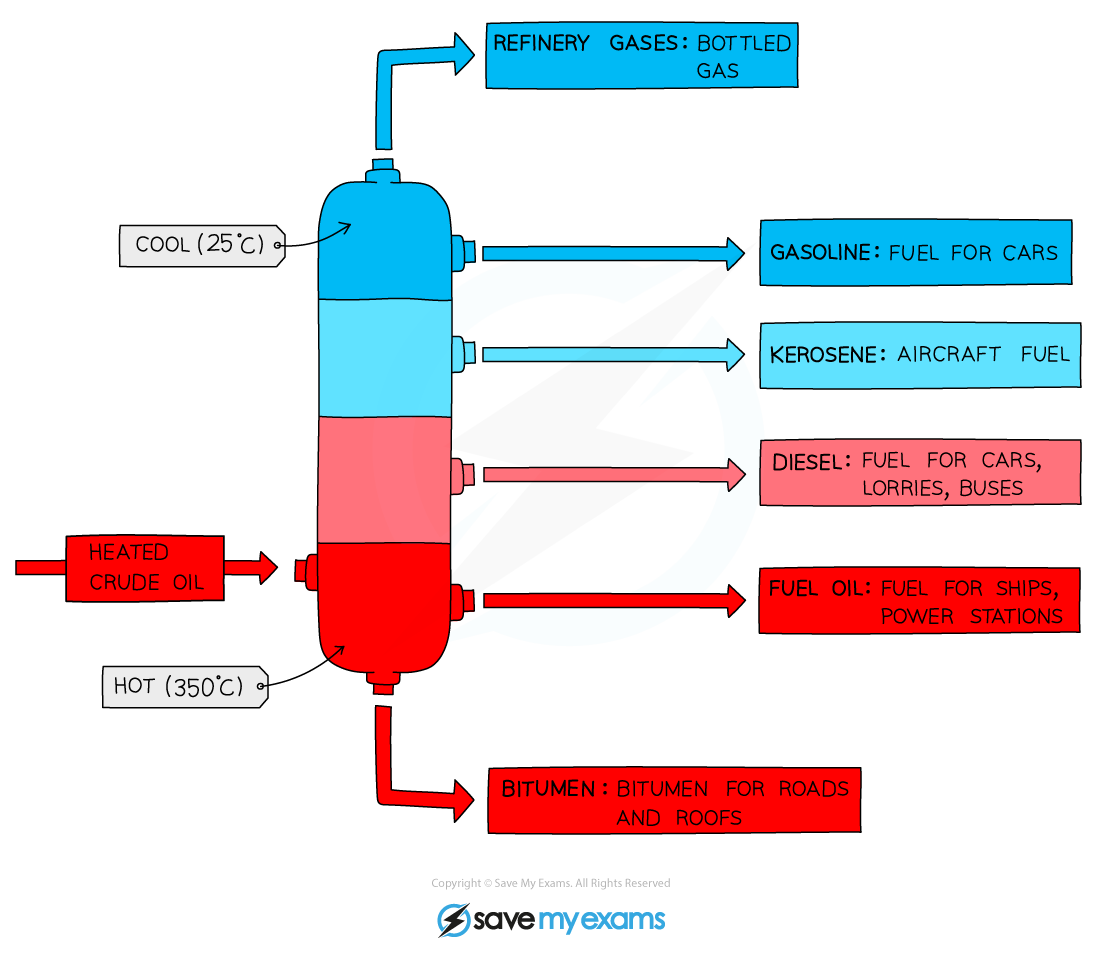
why is cracking sometimes used instead of fractional distillation
However, the smaller hydrocarbon fractions (such as gasoline fractions) are in high demand compared to the larger ones
Therefore, some of the excess heavier fractions are broken down into smaller, more useful compounds
These more useful compounds include alkanes and alkenes of lower relative formula mass (Mr)
This process is called cracking
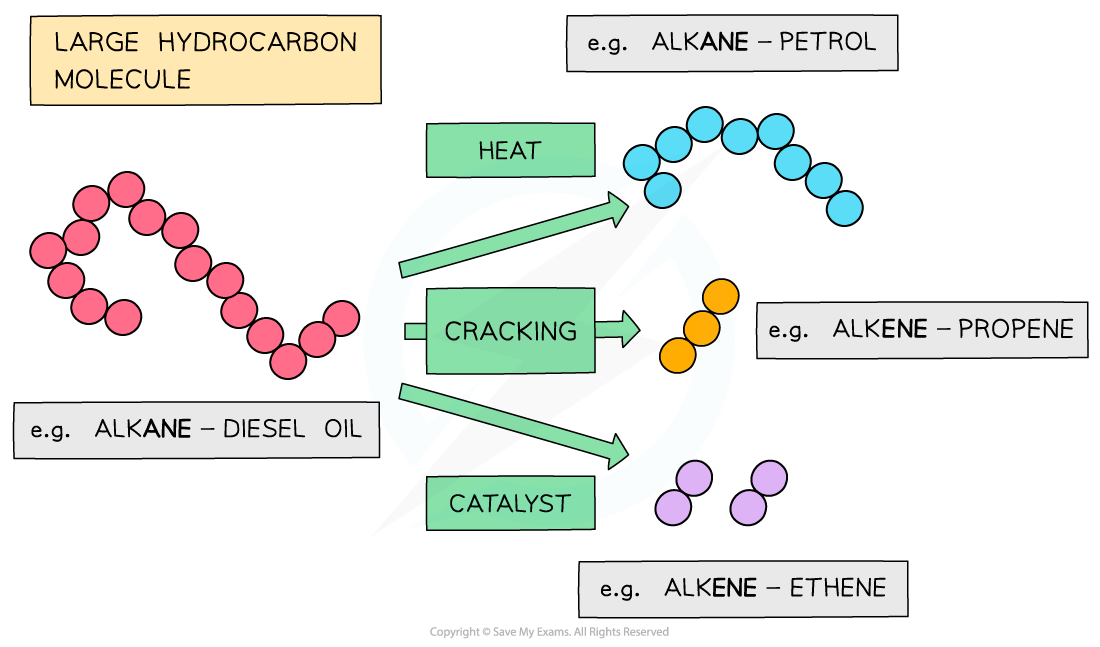
cracking process
The large hydrocarbon molecules are fed into a steel chamber, heated to a high temperature and then passed over an aluminium oxide (Al2O3) catalyst
The chamber does not contain any oxygen to prevent combustion of the hydrocarbon to water and carbon dioxide
When a large hydrocarbon is cracked, a smaller alkane and alkene molecules are formed
E.g. octane and ethene from decane
Cracking of long-chain hydrocarbons

low-molecular mass alkanes and alkenes formed from cracking
The low-molecular mass alkanes formed make good fuels and are in high demand
The low-molecular mass alkenes are more reactive than alkanes due to their double bond
This makes them useful for the chemical industry as the starting compounds (feedstock) for making new products
E.g. they are used as monomers in polymerisation reactions
Using alkenes to form other useful compounds

strength of c-h bonds in alkanes
Alkanes consist of carbon and hydrogen atoms which are bonded together by single bonds, known as sigma (σ) bonds, formed from the direct overlap of atomic orbitals.
Unless a lot of heat is supplied, it is difficult to break these strong C–C and C–H covalent bonds, which have high bond enthalpies.
This decreases the alkanes’ reactivities in chemical reactions, making them relatively inert under normal conditions.
The electronegativities of the carbon and hydrogen atoms in alkanes are almost the same, with carbon being only slightly more electronegative than hydrogen.
This means that both atoms share the electrons in the covalent bond almost equally, leading to little to no bond polarity.
As a result of this, alkanes are nonpolar molecules and have no partial positive or negative charges (δ+ and δ– respectively), so they do not interact strongly with polar solvents or reagents.
Alkanes therefore do not react with polar reagents, such as acids, bases, or oxidizing agents under standard conditions.
They have no electron-deficient areas to attract nucleophiles, which are species that donate electron pairs.
They also lack electron-rich areas to attract electrophiles, which are species that accept electron pairs.
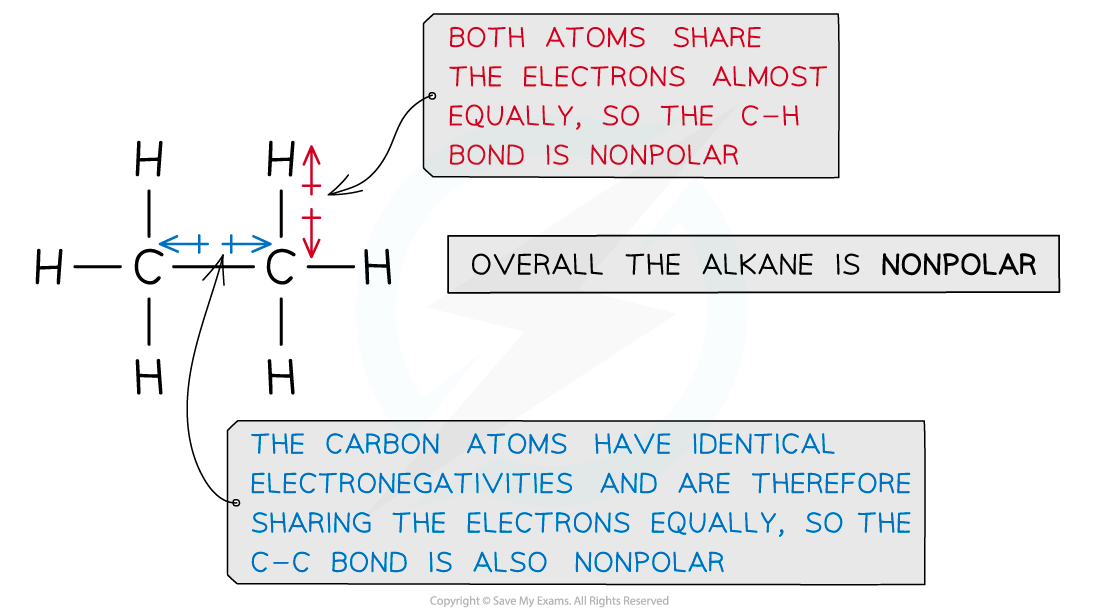
Due to the unreactivity of alkanes, they only react in combustion reactions and undergo substitution by halogens
examiner tips and tricks
Remember: nucleophiles are negatively charged and are attracted to electron-deficient regions
Electrophiles are positively charged and attracted to electron-rich regions
Combustion of Alkanes & the Environment
Cars’ exhaust fumes include toxic gases such as carbon monoxide (CO), oxides of nitrogen (NO/NO2) and volatile organic compounds (VOCs)
When released into the atmosphere, these pollutants have drastic environmental consequences damaging nature and health
carbon monoxide and its effects
When oxygen supply is limited, carbon monoxide (CO) forms instead of carbon dioxide:
fuel + oxygen → carbon monoxide + water
For example, Incomplete combustion of propane:
propane + oxygen → carbon monoxide + water
C3H8 (l) + 3½O2 (g) → 3CO (g) + 4H2O (l)
Carbon monoxide is extremely dangerous because it is:
Colourless and odourless (can’t be seen or smelled)
Hard to detect without a sensor
It is a toxic and poisonous gas that binds irreversibly to haemoglobin in the blood.
This prevents haemoglobin from carrying oxygen.
Lack of oxygen transport leads to:
Dizziness
Loss of consciousness
Potentially death if not treated
The effect of carbon monoxide on haemoglobin
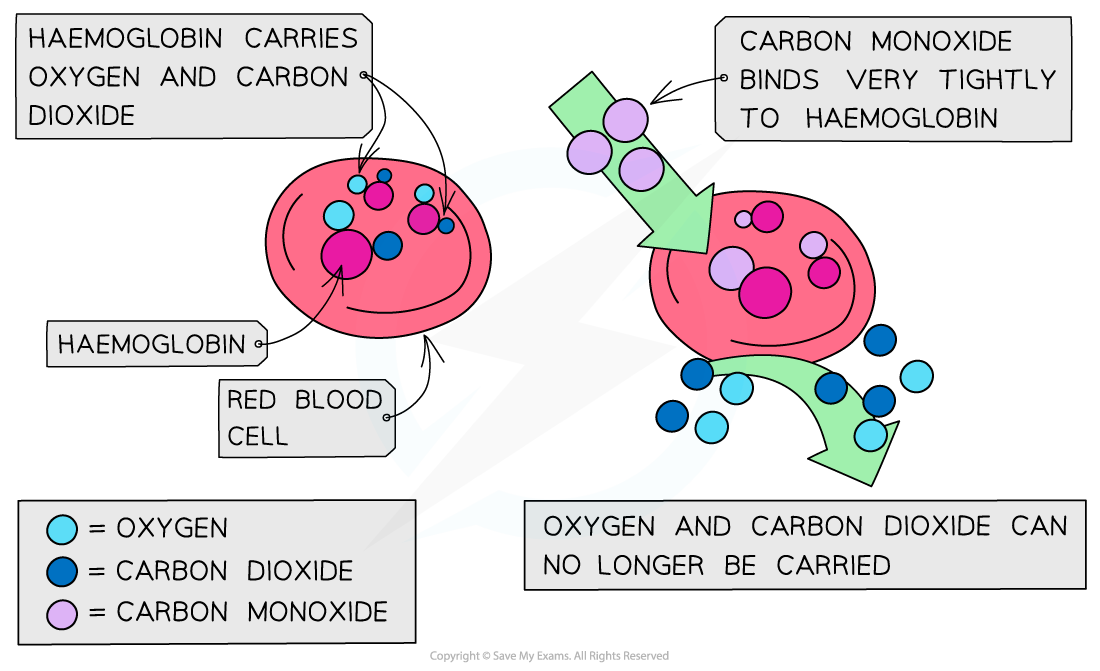
The high affinity of CO to haemoglobin prevents it from binding to O2 and CO
oxides of nitrogen
Normally, nitrogen is too unreactive to react with oxygen in air
In a car’s engine, high temperatures and pressures are reached, causing the oxidation of nitrogen to take place:
N2 (g) + O2 (g) → 2NO (g)
N2 (g) + 2O2 (g) → 2NO2 (g)
The oxides of nitrogen are then released in the car’s exhaust fumes into the atmosphere
Car exhaust fumes also contain unburnt hydrocarbons from fuels and their oxides(VOCs)
In the air, the nitrogen oxides can react with these VOCs to form peroxyacetylnitrate (PAN) which is the main pollutant found in photochemical smog
PAN is also harmful to the lungs, eyes and plant life
Nitrogen oxides can also dissolve and react in water with oxygen to form nitric acid which is a cause of acid rain
Acid rain can cause corrosion of buildings, endanger plant and aquatic life (as lakes and rivers become too acidic) and directly damage human health
catalytic converters
To reduce the amount of pollutants released in cars’ exhaust fumes, many cars are now fitted with catalytic converters
Precious metals (such as platinum) are coated on a honeycomb to provide a large surface area
The reactions that take place in the catalytic converter include:
Oxidation of CO to CO2:
2CO + O2 → 2CO2
or
2CO + 2NO → 2CO2 + N2
Reduction of NO/NO2 to N2:
2CO + 2NO → 2CO2 + N2
Oxidation of unburnt hydrocarbons:
CnH2n+2 + (3n+1)[O] → nCO2 + (n+1)H2O
Pollutants, their effect & removal summary
Carbon Monoxide (CO)
Formation:
Incomplete combustion of alkanes in car engines
Environmental consequence:
Toxic gas
Catalytic removal:
Oxidised to carbon dioxide (CO2):
2CO + O2 → 2CO2
2CO + 2NO → 2CO2 + N2
Oxides of Nitrogen (NO, NO2 etc.)
Formation:
Oxidation of nitrogen in car engines (due to high temperatures)
Environmental consequence:
Dissolve in water and react with oxygen to form acid rain
Catalytic removal:
Reduced to nitrogen gas:
2CO + 2NO → 2CO2 + N2
Volatile Organic Compounds (VOCs)
Formation:
Unburnt hydrocarbons from fuels
Oxides of these hydrocarbons formed in car engines
Environmental consequence:
React with oxides of nitrogen in the atmosphere to form PAN
Catalytic removal:
Oxidised to CO2 and H2O
General formula reaction:
CnH2n+2 + (3n + 1)[O] → nCO2 + (n + 1)H₂O
PAN (peroxyacyl nitrate)
Formation:
From the photochemical reaction of VOCs and nitrogen oxides in the atmosphere
Environmental consequence:
Contributes to photochemical smog
Catalytic removal:
Oxidise unburnt hydrocarbons
Reduce nitrogen oxides to prevent PAN formation
examiner tips and tricks : Although CO2 is not a toxic gas, it is still a pollutant causing global warming and climate change
-