Biochem - 6.4/7.1 (lect + book notes)
1/486
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
487 Terms
Q: What is the sliding filament model?
A: A model stating that actin and myosin filaments slide over one another during muscle contraction using chemical energy from ATP hydrolysis.
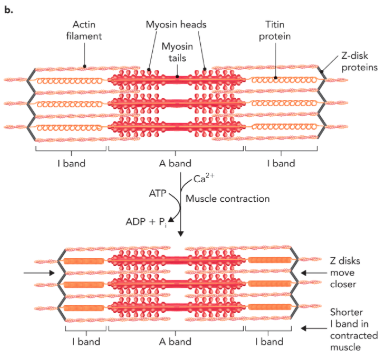
Q: What does Figure 6.61 show about sarcomeres?
A: Electron micrograph of a sarcomere with two Z disks, two I bands, and an A band. During contraction, thick and thin filaments slide past each other, shortening distance between Z disks. The A band width stays constant, while I bands shorten.
Q: What initiates muscle contraction?
A: Neuronal stimulation at neuromuscular junctions releases Ca²⁺ from the sarcoplasmic reticulum.
Q: How does Ca²⁺ trigger filament movement?
A: Ca²⁺ binding and ATP hydrolysis drive conformational changes in thin and thick filaments, causing sliding and movement.
Q: What happens to Z disks during contraction?
A: They are pulled closer together as thick and thin filaments slide; titin “springs” compress.
Q: What band changes during contraction?
A: The I band decreases in width, but the A band remains constant.
Q: How do Ca²⁺ binding and ATP hydrolysis cause contraction?
A: They allow thick and thin filaments to move past one another, producing contraction by structural changes in skeletal muscle proteins.
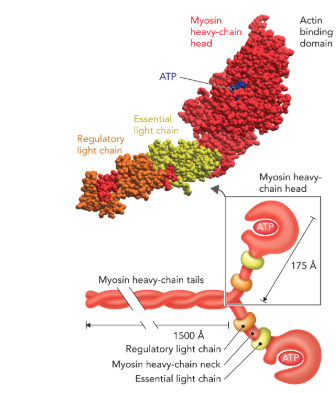
Q: What does Figure 6.62 show about the myosin complex?
A: Myosin in thick filaments has 6 polypeptide chains: myosin, regulatory light chain, essential light chain. Structural regions: tail (coiled coil), head (globular, binds/hydrolyzes ATP, binds actin), neck (connects head and tail).
Q: How are thick filaments formed?
A: Tails of myosin polypeptides coil together, forming bundles of thick filaments.
Q: What happens when ATP is hydrolyzed by myosin heads?
A: It causes conformational changes that drive filament sliding and contraction.
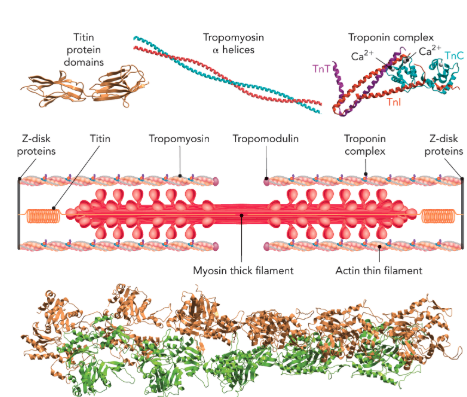
Q: What does Figure 6.63 show about thin filaments?
A: Actin forms right-handed polymers capped by tropomodulin. Troponin complex (TnT, TnI, TnC) associates with tropomyosin and regulates contraction. TnC binds Ca²⁺, controlling contraction. Also shows titin spring domains.
Q: What is titin and its role?
A: A gigantic protein acting as a spring, changing length up to 10-fold during contraction, anchoring myosin to Z-disks. It’s the longest known polypeptide (~34,350 amino acids, >3 million Da).
Q: How do myosin heads move filaments?
A: They bind and release actin subunits, undergoing conformational ratchet-like changes driven by Ca²⁺ and nucleotides.
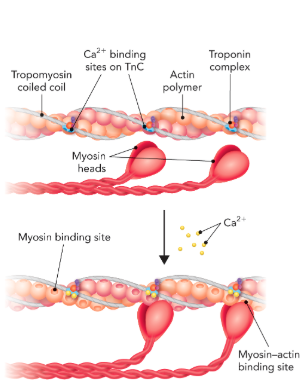
Q: What does Figure 6.64 show?
A: Calcium binds troponin, causing conformational changes that shift tropomyosin, exposing actin’s myosin binding sites → enabling contraction.
Q: How do tropomyosin and troponin regulate contraction?
A: At rest, tropomyosin blocks actin binding sites. With Ca²⁺, troponin shifts tropomyosin away, allowing myosin to bind actin.
Q: What drives the actin–myosin reaction cycle?
A: ATP binding, hydrolysis, and release.
Q: What are the five steps of the actin–myosin cycle?
Myosin (ADP + Pi bound) binds actin.
Release of Pi induces power stroke (filament slides).
ADP release empties binding site.
New ATP binds, causing myosin to release actin.
ATP hydrolysis resets myosin head.
Q: How far does each power stroke move actin?
A: ~70 Å toward the sarcomere center.
Q: What are muscle cells made of?
A: Large fused cells called myoblasts, containing many nuclei and a plasma membrane (sarcolemma). Inside are myofibrils, surrounded by sarcoplasmic reticulum.
Q: What structures allow O₂ and nutrient exchange in muscle?
A: T-tubule invaginations of the sarcolemma.
Q: What transporter modulates Ca²⁺ in muscle?
A: SERCA (sarcoplasmic reticulum Ca²⁺ ATPase).
Q: What are myofibrils made of?
A: Interlocking thick (myosin) and thin (actin, tropomyosin, troponin) filaments arranged into repeating sarcomeres.
Q: What is a sarcomere?
A: The repeating unit of skeletal muscle bounded by Z disks, decreasing in length during contraction.
Q: What do Z disks do?
A: Anchor thin and thick filaments, pulling closer together during contraction.
Q: How many thin filaments can a thick filament interact with?
A: Up to 6 thin filaments.
Q: How many thick filaments can a thin filament contact?
A: Up to 3 thick filaments.
Q: What are thick filaments made of?
A: Hundreds of myosin molecules arranged with tails in the center, heads at the ends.
Q: What are thin filaments made of?
A: Actin polymers (~375 aa residues), tropomyosin (α-helical coiled coil), and troponin complex.
Q: What is the troponin complex composed of?
A:
TnT: binds tropomyosin.
TnI: inhibits myosin-actin binding.
TnC: binds Ca²⁺, controls contraction.
Q: What protein connects myosin to Z-disks and acts as a spring?
A: Titin.
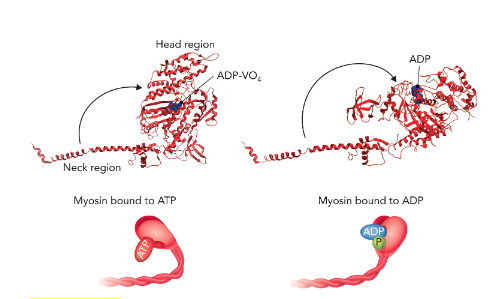
Q: What does Figure 6.66 show about the myosin head?
A: The conformation of the myosin head and neck region changes depending on nucleotide state. With ADP·VO₄ (mimic of ADP + Pi), the head/neck adopt one conformation; with ADP, they adopt another. These represent two states in the actin–myosin cycle.
Q: What are the five steps of the actin–myosin reaction cycle?
Ca²⁺ changes thin filament → myosin heads (ADP + Pi bound) bind actin with high affinity.
Pi release → large conformational change (power stroke), sliding actin along myosin.
ADP release frees the nucleotide binding site.
New ATP binds → myosin disengages from actin.
ATP hydrolysis resets myosin head into ADP + Pi form, ready for next cycle.
Q: What triggers strong binding of myosin heads to actin?
A: Ca²⁺-induced changes in thin filament structure and myosin heads containing ADP + Pi.
Q: What causes the power stroke in the actin–myosin cycle?
A: Release of inorganic phosphate (Pi) from the myosin head.
Q: What step allows myosin to release from actin?
A: Binding of a new ATP molecule to the myosin head.
Q: What resets the myosin head for another contraction cycle?
A: ATP hydrolysis, producing the ADP + Pi form.
Q: When does muscle relaxation occur?
A: When neuromuscular signals stop and cytosolic Ca²⁺ decreases due to SERCA-mediated active transport.
Q: What happens to actin binding sites during relaxation?
A: They are blocked again as Ca²⁺ levels fall.
Q: How do filaments move during relaxation?
A: Thick and thin filaments slip in the opposite direction, titin “uncoils,” and the sarcomere elongates.
Q: When was the discovery made that proteins can function as enzymes?
A: In the 1930s, nearly 100 years after proteins were first characterized. They were once called ferments of zymes (from yeast).
Q: Who first provided evidence that enzymes are proteins?
A: James Sumner in 1926, when he purified and crystallized urease from jack beans, showing urea hydrolysis was mediated by a protein enzyme.
Q: Why was crystallization of urease important?
A: It demonstrated protein purity and proved proteins could act as enzymes (Figure 7.1).
Q: What confirmed Sumner’s discovery?
A: John Northrop’s purified pepsin preparation, which validated that proteins can function as enzymes.
Q: How does modern understanding of enzyme catalysis build on early studies?
A: By analyzing 3D protein structures in the context of enzyme kinetics.
Q: Why doesn’t 3D structure alone explain enzyme function?
A: It does not reveal the dynamic processes and conformational changes required for catalysis.
Q: What was the lock-and-key model of enzyme specificity?
A: Proposed by Emil Fischer in 1894, it explained specificity by rigid complementarity between substrate and enzyme.
Q: What was the limitation of the lock-and-key model?
A: It could not explain enzyme regulation or binding to sites buried deep inside proteins.
Q: What model replaced the lock-and-key theory in 1958?
A: The induced-fit model proposed by Daniel Koshland.
Q: What is the induced-fit model?
A: The enzyme is flexible (like a glove) and molds around the substrate, allowing more weak interactions and catalysis.
Q: What is the advantage of the induced-fit model?
A: Permits more weak interactions, structural adjustments, and stable enzyme–substrate complexes, enhancing catalysis.
Q: What is conformational selection?
A: A model where proteins sample many conformations; ligand binding shifts equilibrium toward the preferred binding state, followed by induced fit.
Q: What are the three critical aspects of enzymes?
A: 1) Substrate binding specificity, 2) Structural changes upon binding, 3) Enzyme activity regulation.
Q: What do enzyme active sites provide?
A: A protective microenvironment away from bulk solvent where catalysis occurs (Figure 7.2).
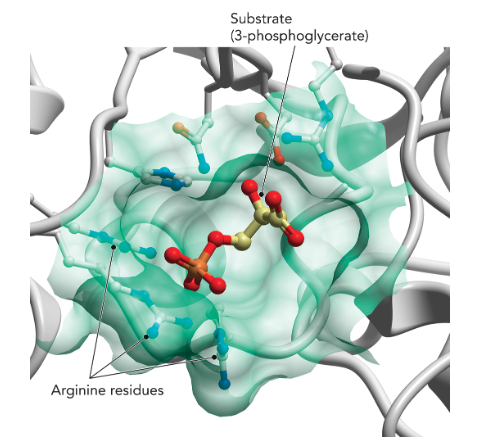
Q: What interactions stabilize substrate binding?
A: Hydrogen bonds, ionic interactions, and van der Waals forces.
Q: What classic example demonstrates induced-fit substrate binding?
A: Hexokinase binding glucose and excluding H₂O to facilitate ATP phosphorylation (Figure 7.3).
Q: What happens to hexokinase upon glucose binding?
A: A large conformational change blocks water and allows glucose phosphorylation.
Q: How is enzyme activity regulated in cells?
A: By bioavailability (amount present, controlled by gene expression and turnover) and activity (regulation via covalent modification or regulatory molecules).
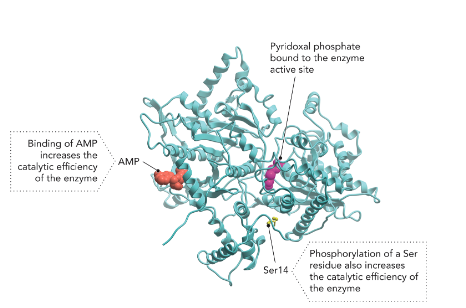
Q: What is an example of enzyme regulation?
A: Glycogen phosphorylase activity, regulated by phosphorylation of Ser/Thr/Tyr residues and binding of AMP.
Q: What is glycogen phosphorylase’s role?
A: Controls glucose release from glycogen in liver and muscle, regulating energy needs (Figure 7.4).
Q: How do enzymes act as catalysts?
A: By increasing the rate of reactions (forward and reverse) without changing equilibrium or ΔG.
Q: (Example) What is the reaction for hydrogen peroxide decomposition?
A: 2 H₂O₂ ⇌ 2 H₂O + O₂
Q: Why does H₂O₂ decompose so slowly at room temp?
A: Without a catalyst, 1 mol would take ~3 years to decompose halfway (t½).
Q: How can the decomposition rate of H₂O₂ be increased?
A: By adding free iron (Fe²⁺/Fe³⁺), reducing t½ to ~11.6 minutes.
Q: Why is H₂O₂ dangerous in cells?
A: It is reactive and toxic; must be eliminated quickly.
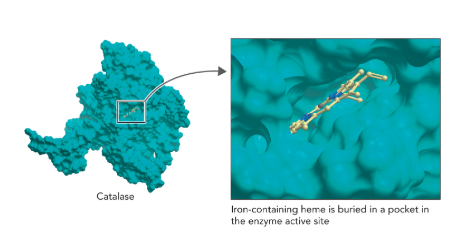
Q: What enzyme detoxifies H₂O₂ in cells?
A: Catalase, which decomposes H₂O₂ into water and oxygen extremely efficiently (Figure 7.5).
Q: What is the rate of catalase activity?
A: Millions of H₂O₂ molecules decomposed per second per catalase molecule.
Q: How much faster is catalase than the uncatalyzed reaction?
A: ~10¹⁵ times faster.
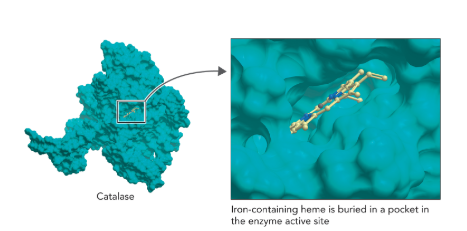
Q: What does Figure 7.5 show about catalase’s mechanism?
A: Catalase uses a heme group with Fe in a porphyrin ring; Fe cycles between Fe³⁺ and Fe⁴⁺, aided by amino acids for proton movement and electron transfer.
Q: What does the transition state theory (Eyring, 1930s) state?
A: Substrate-to-product conversion requires a high-energy transition state in which a molecule can either remain substrate or become product.
Q: How long do molecules remain in the transition state?
A: ~10⁻¹⁵ seconds (the time of a single atomic vibration).
Q: Why are molecules in the transition state not isolatable intermediates?
A: They are transient, unstable molecules at a required energy level for conversion.
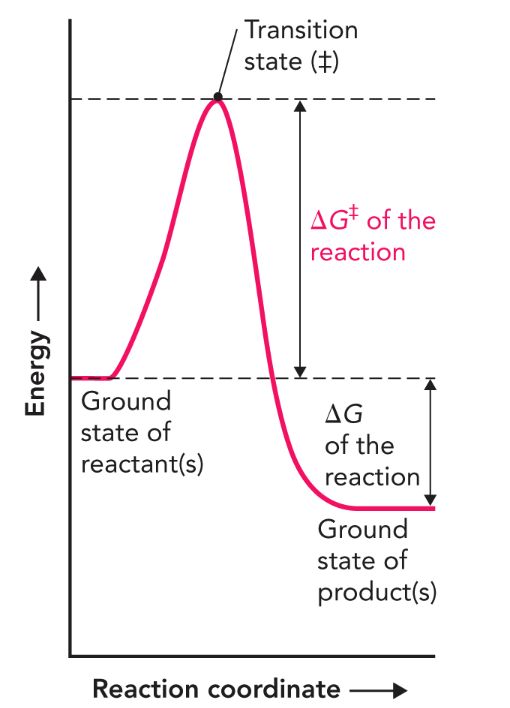
Q: What does Figure 7.6 describe?
A: Free energy of a chemical reaction using a reaction coordinate diagram.
Q: What is ΔG of a reaction?
A: The free energy change between ground state reactants and products; unaffected by catalysts.
Q: What is ΔG‡ (activation energy)?
A: The difference in energy between the ground state and the transition state, which must be overcome to form product.
Q: What does the “double dagger” (‡) symbol represent?
A: The transition state energy barrier.
Q: How do enzymes increase reaction rate?
A: By lowering ΔG‡, thus lowering the transition energy barrier.
Q: What happens in an uncatalyzed reaction?
A: Molecules must collide with proper orientation and enough energy to form new bonds (Figure 7.7a).
Q: What role does enzyme active site play in catalysis?
A: Provides optimal environment, reducing reliance on chance collisions, and lowering ΔG‡.
Q: Why is catalase an excellent catalyst?
A: It provides a reactive Fe³⁺ porphyrin ring in a protected environment, reducing ΔG‡ from +71 kJ/mol to +8 kJ/mol and increasing rate 10¹⁵-fold (Figure 7.7b).
Q: What is the ΔG of H₂O₂ decomposition?
A: Approximately –95 kJ/mol (highly favorable regardless of catalyst).
Q: How does catalase affect the rate of H₂O₂ decomposition?
A: It dramatically increases the rate by lowering the transition state barrier, making it easier for H₂O₂ to convert.
Q: What are cofactors?
A: Non-protein molecules that aid enzymes in catalysis by supplementing amino acid chemistry.
Q: What is a holoenzyme vs. an apoenzyme?
A: Holoenzyme = enzyme + cofactor (active); Apoenzyme = enzyme without cofactor (inactive).
Q: Give examples of inorganic cofactors.
A: Fe²⁺, Mg²⁺, Mn²⁺, Cu²⁺, Zn²⁺.
Q: What is the role of Zn²⁺ in proteases?
A: Facilitates deprotonation of water, making it a nucleophile.
Q: Why is mercury (Hg²⁺) toxic to enzymes?
A: It can replace Zn²⁺ in enzymes in a nonproductive way.
Q: What role does Mg²⁺ play?
A: Stabilizes negatively charged phosphoryl groups in ATP hydrolysis.
Q: How does Cu²⁺ function as a cofactor?
A: Coordinates with His residues in enzymes like Alcaligenes faecalis nitrite reductase, aiding nitrite reduction to nitric oxide.
Q: What are coenzymes?
A: Organic cofactors that provide additional chemical flexibility in catalysis.
Q: What are prosthetic groups?
A: Coenzymes permanently associated with enzymes (e.g., heme in catalase).
Q: What vitamins are common sources of coenzymes?
A: Niacin (vitamin B₃ → NAD⁺/NADH) and Thiamine (vitamin B₁ → thiamine pyrophosphate).
Q: What disease results from vitamin B₁ deficiency?
A: Beriberi, with neurological symptoms.
Q: What is the role of thiamine pyrophosphate?
A: Coenzyme in decarboxylation reactions (e.g., pyruvate dehydrogenase complex).
What does lactate dehydrogenase do?
A: Converts pyruvate → lactate during anaerobic respiration, oxidizing NADH to NAD⁺.
Q: What role does NADH/NAD⁺ play in redox reactions?
A: Acts as an electron carrier, donating/accepting electron pairs during enzyme catalysis.
Q: Why are NAD⁺/NADH called co-substrates?
A: They are altered during reactions (oxidized/reduced) and must be regenerated later.
Q: What are some coenzymes covalently linked to enzymes?
A: Coenzymes like lipoic acid can attach to amino acid residues (e.g., lysine), serving as integral catalytic components in redox and acyl transfer reactions.
Q: What are the oxidized and reduced forms of the coenzyme derived from lipoic acid?
A: Oxidized form = lipoamide, reduced form = dihydrolipoamide (Figure 7.10).
Q: What role does lipoamide play in the pyruvate dehydrogenase complex?
A: It participates in acyl transfer reactions, transferring the acetyl group to form acetyl-CoA.
Q: What other coenzymes are required by the pyruvate dehydrogenase complex?
A: Flavin adenine dinucleotide (FAD), coenzyme A, and thiamine pyrophosphate (TPP).