SECTION 03: ENZYME REGULATION
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
🧪 Why regulate enzymes?
⚖ What happens if enzymes aren't regulated?
❌ Wasted energy and resources (making stuff the cell doesn’t need)
❌ Shortages of key molecules the cell does need
⚠ Can disrupt metabolism and harm the cell
🔄 How is enzyme activity controlled?
Through regulation methods (explored in upcoming slides)
Influenced by environmental and physical factors (like pH, temperature)
✨ Summary:
Enzymes must be carefully regulated so cells don’t overproduce or underproduce important molecules. This helps keep metabolism efficient and balanced.
🌡 PHYSICAL FACTORS THAT AFFECT ENZYME RATE
TEMPERATURE
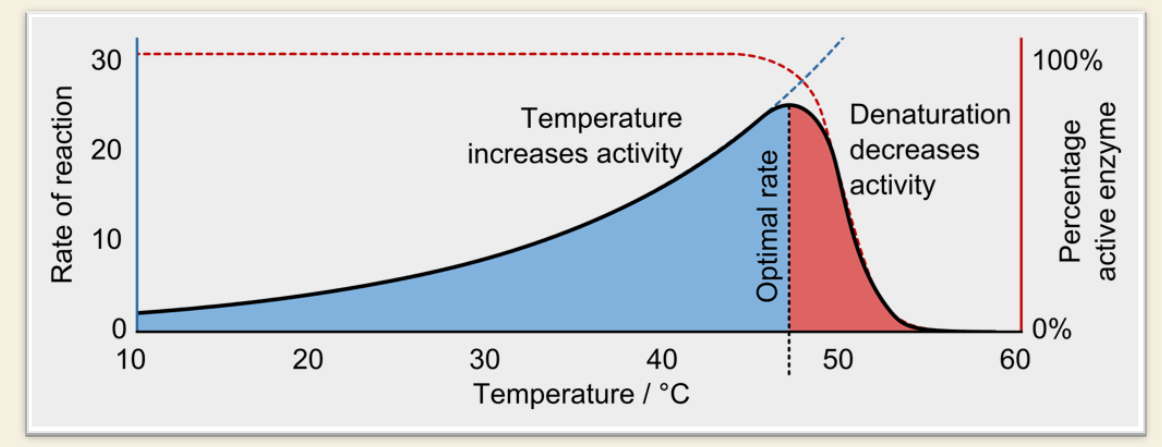
✅ What happens as temperature increases?
Enzyme activity increases (molecules move faster → more collisions)
⚠ What is the optimal range?
Most enzymes work best at 30–37°C (human body range)
❌ What happens if it's too hot?
The enzyme denatures (loses shape)
This causes a sharp drop in activity
🖼 Graph 1 Key Points:
Blue zone = temperature is helping reaction rate
Red zone = enzyme is denaturing = sharp drop in activity
Optimal temperature = peak of the curve (~around 40-45°C in the image)
Dashed red line = % of active enzyme decreases after denaturation begins
⚗ pH
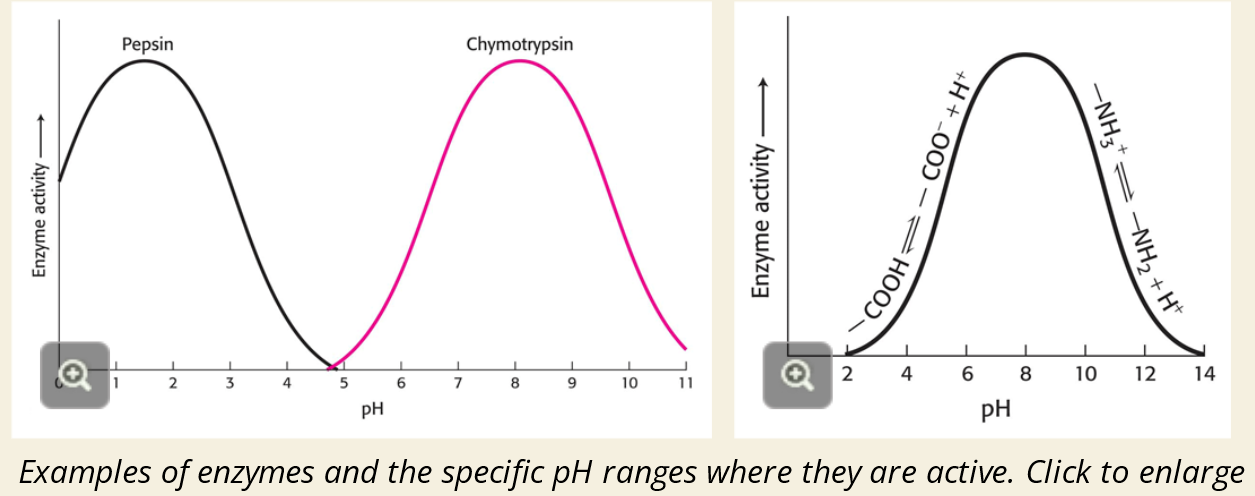
✅ Why does pH matter?
pH affects the charges on amino acids in the enzyme
These charges are crucial for proper folding and reaction at the active site
❌ What happens if pH is too high or too low?
Enzyme unfolds or stops working
This is called denaturation
🖼 Graph 2 Key Points (Pepsin vs. Chymotrypsin):
Pepsin works best at very low pH (~2) → stomach acid
Chymotrypsin works best at neutral to slightly basic pH (~8) → small intestine
🖼 Graph 3 Key Point (Charge-based pH activity):
Activity depends on how amino acids are ionized
Proper charges = enzyme works
Wrong pH = changes ionization → enzyme loses function
✨ Summary:
Enzymes work best in specific temperature and pH ranges. Too high or too low in either can lead to loss of shape and function. Each enzyme has its own optimal pH and temperature based on where it normally works in the body.
⚙ What is a substrate?
📈 What happens as substrate concentration increases?
More substrate = faster reaction rate (to a point)
At very high concentrations, enzyme becomes saturated → reaction hits maximum speed (Vmax)
⚖ What about reversible reactions?
Reaction will reach equilibrium between substrate and product
But if the product is removed (e.g., used in another reaction), the forward reaction keeps going
🍬 A CLOSER LOOK: Glycogen Breakdown (Image)
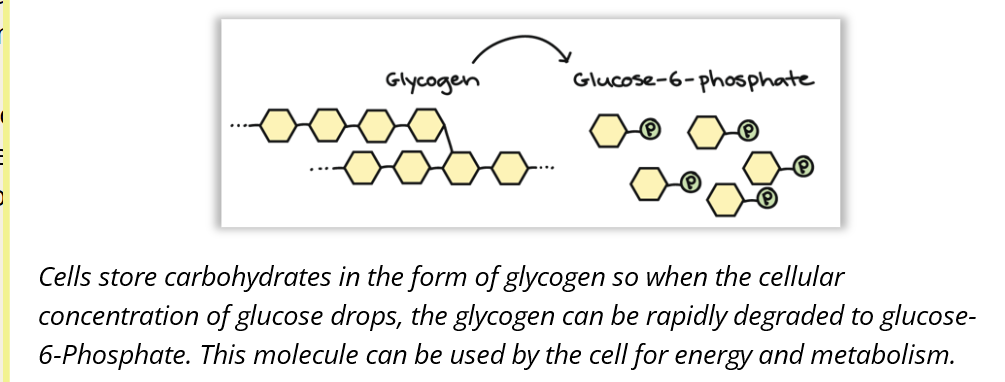
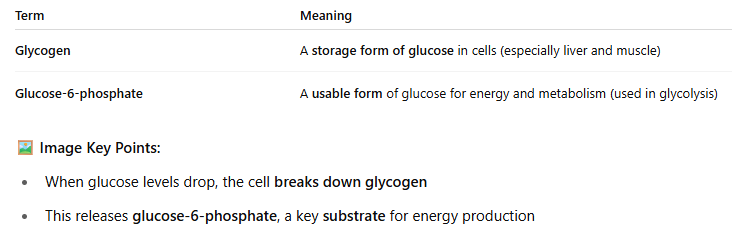
✨ Summary:
Enzyme activity depends on substrate availability. If there's not enough, the reaction slows. The body stores some substrates (like glycogen) and can release them when needed to keep metabolism going.
❓ What is product inhibition?
When the product of a reaction binds to the enzyme, blocking the substrate
This slows or reverses the reaction
⚖ Reaction Direction Depends On:
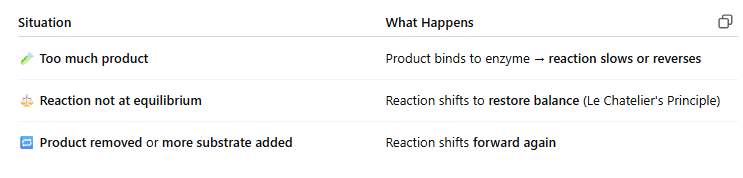
🧩 IMAGE KEY POINTS:

◀ Reverse Reaction Favored:
Reaction: E + P ← ES ← E + S
Happens when:
Product concentration is high
Substrate concentration is low
Forward and reverse rates are similar
▶ Forward Reaction Favored:
Reaction: E + S → ES → E + P
Happens when:
Forward rate (k₁, k₂) is much faster
Product doesn’t build up fast enough to block the enzyme
🔑 Rate Constants:
k₁ / k₂ = forward rate
k₋₁ / k₋₂ = reverse rate
The bigger the forward rate compared to reverse, the more likely the reaction goes forward
✨ Summary:
If product builds up, it can bind to the enzyme and push the reaction backward. But if product is removed or more substrate is added, the reaction moves forward again. This is a fast, reversible way to regulate enzyme activity.
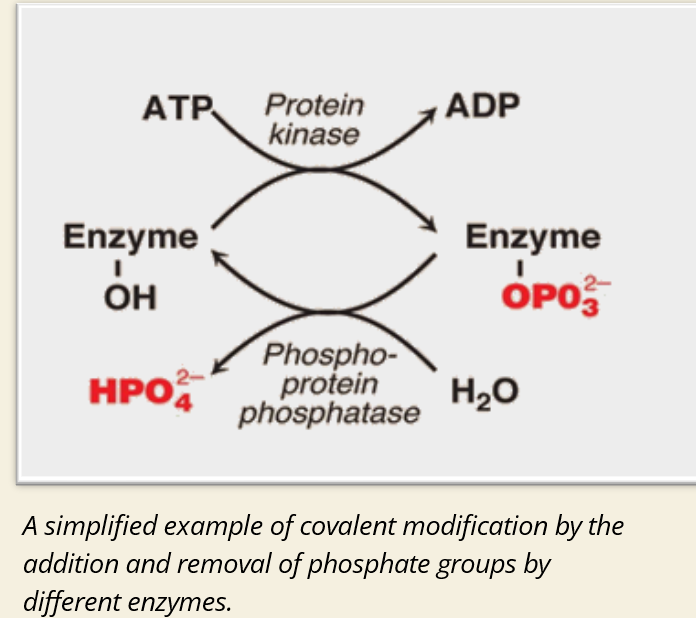
🧪 What is covalent modification?
A chemical group is added or removed from an enzyme
This changes the enzyme’s shape or charge → affects activity
⚡ Common types of covalent modification:
Phosphorylation (adding a phosphate group)
Dephosphorylation (removing a phosphate group)
Glycosylation (adding sugar)
Adding prosthetic groups (like metals or cofactors)
🔁 What happens during phosphorylation?
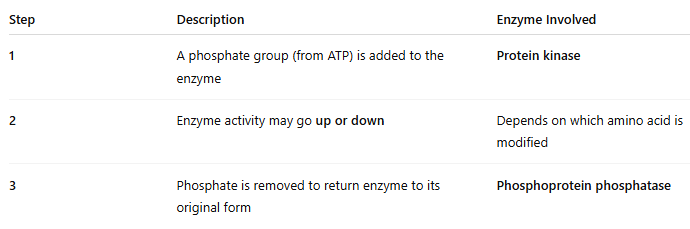
🖼 IMAGE KEY POINTS:
Left side = inactive enzyme with –OH group
Protein kinase adds phosphate → ATP → ADP
Enzyme becomes phosphorylated → may become active or inactive
Phosphatase removes phosphate → returns enzyme to –OH form
This process is reversible and enzyme-specific
🧬 Where does phosphorylation occur?
On amino acids with –OH groups:
Serine
Threonine
Tyrosine
✨ Summary:
Covalent modification like phosphorylation is a way to quickly turn enzymes on or off. It’s done by specific enzymes (kinases and phosphatases) and is a common form of cell signaling and regulation.
❓ What are most enzymes like?
Most are Michaelis-Menten enzymes
They work only when substrate is available
They are not regulated by the cell (just run when needed)
🔐 What are allosteric enzymes?
Special enzymes with:
Multiple active sites (bind substrate)
Regulatory sites (bind activators/inhibitors)
These enzymes control the flow through important pathways
allosteric enzymes - 📍 Why are they important?
They regulate the committed step = the step that locks the pathway in
Once that step happens → the rest of the pathway must continue
allosteric enzymes - 🔄 How are they regulated?
By:
Products of the same pathway (feedback inhibition)
Products from other pathways (cross-pathway regulation)
✨ Summary:
Allosteric enzymes act like gatekeepers. They control key pathway steps by responding to signals from other molecules, helping the cell avoid waste and respond to changes.
🧩 What is an allosteric enzyme?
An enzyme that has extra binding sites called allosteric sites
These sites control enzyme activity (turn it on or off)
🧪 What binds to allosteric sites?
Effectors (small molecules that regulate activity)
Can be activators or inhibitors
Don’t bind to the active site, but change enzyme shape
🎯 What happens when an effector binds?
Inhibitor binds → enzyme shape changes → active site blocked
Inhibitor leaves → enzyme returns to normal → substrate can bind again
🔁 Enzyme shapes:
Enzymes naturally switch between:
Active form = can bind substrate
Inactive form = can’t bind substrate
Substrate binding stabilizes the active form
👯 Multimeric enzymes (with multiple subunits):
Binding at one active site can affect the other = cooperativity
Example: Binding at site A makes site B more likely to bind
🧬 Allosteric regulation happens in:
Enzymes
Transporters (like channels)
Proteins like hemoglobin (not enzymes, but still regulated this way)
🎥 Video Key Concept:
Inhibitor binding → locks enzyme in inactive shape
Substrate binding → locks enzyme in active shape
This is reversible – enzyme toggles between forms
🧠 2 Types of Allosteric Effectors:
Homotropic = the substrate itself acts as an effector
Heterotropic = a different molecule (not the substrate) regulates activity
✨ Summary:
Allosteric enzymes have special control sites that change enzyme shape and function. This allows fast, reversible regulation, helping the cell respond to changes quickly and efficiently.
🧬 What is a homotropic effector?
A substrate that also acts as a regulator
Binds to one active site and changes the shape of the enzyme → affects other sites
Usually positive → makes it easier for more substrates to bind
🎯 Example: Hemoglobin (not an enzyme, but same principle)
Oxygen (O₂) is a homotropic effector
When one oxygen binds → hemoglobin changes shape → other O₂ sites bind more easily
This is called cooperative binding
🧩 What’s happening in the image?
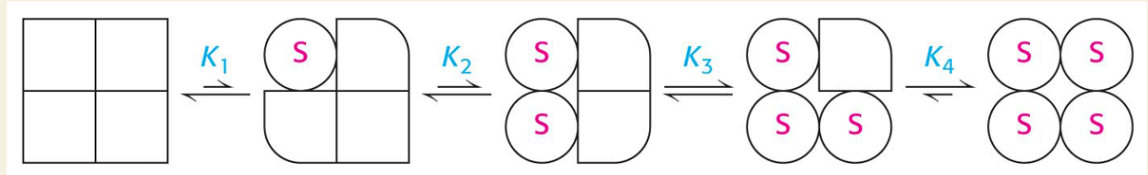

💡 What is cooperativity?
The concept that binding one substrate makes binding others easier
Enzyme becomes more active with each step
✨ Summary:
Homotropic effectors are substrates that help the enzyme become more efficient at binding more of the same substrate. This creates a positive feedback loop within the enzyme, often leading to faster and stronger responses.
❓ What is a heterotropic effector?
A molecule that is NOT the enzyme's substrate
It binds to a regulatory (allosteric) site and changes enzyme activity
Can be:
Positive (+) → increases activity
Negative (–) → decreases activity
🔁 Why are they important?
Usually downstream products of a pathway
Provide feedback regulation:
If too much product builds up → slows down early steps
Keeps cell from wasting energy or overproducing
🧠 IMAGE EXPLAINED:
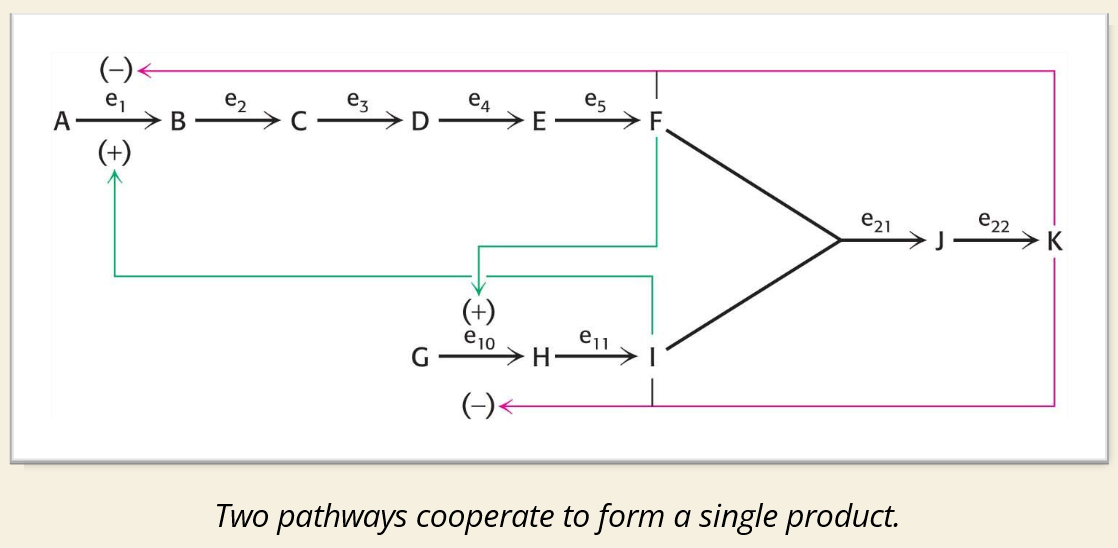

🔍 Regulation in the Image:
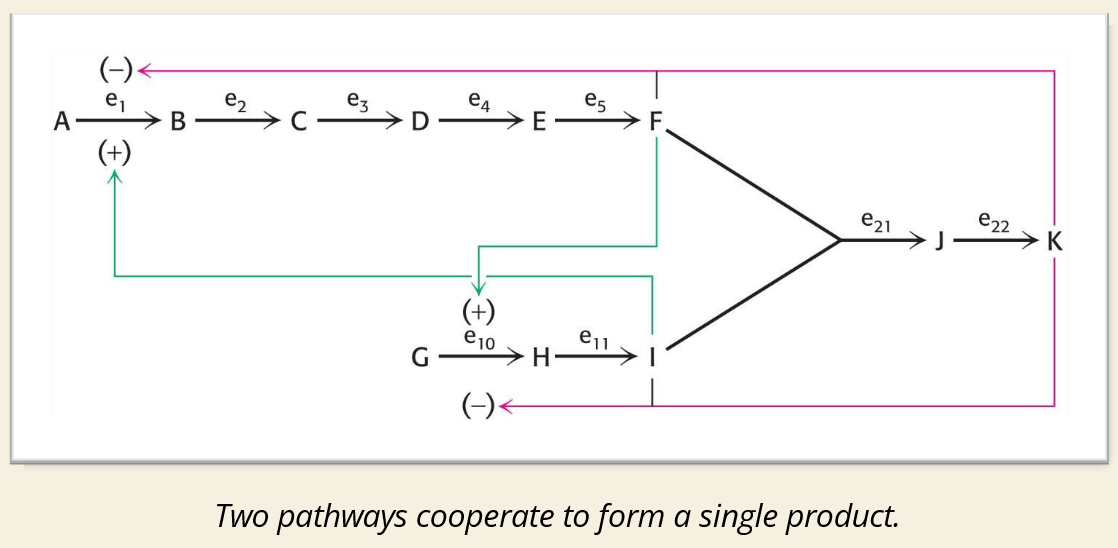
(+) Green Arrows = Activation
Product(s) from later steps stimulate earlier steps
Example: F activates G → I
(–) Pink Arrows = Inhibition
Final product (like K) inhibits enzymes early in the pathway (e.g., E₁ and E₁₁)
Classic negative feedback loop
📌 Enzyme Notation:
e₁, e₂...e₂₂ = Enzymes for each step
Inhibition/activation affects how fast the pathway flows
✨ Summary:
Heterotropic effectors are molecules (usually products) that regulate enzymes at different steps in the pathway. They help balance supply and demand, avoid overload, and fine-tune metabolism.
❓ Why would a cell change how much enzyme it has?
To adjust long-term metabolism
Prevent waste or overproduction
Respond to environmental changes
🧬 Two Main Ways to Regulate Enzyme Amounts:
1. Gene Expression (Enzyme Synthesis)
📖 DNA → mRNA → Protein
The more mRNA is made → the more enzyme is made
The cell can increase or decrease gene expression
🕐 Takes time (hours to days)
⚙ Slower but long-term control
🧬 Two Main Ways to Regulate Enzyme Amounts: 2. Protein Degradation
The cell can break down enzymes when they’re no longer needed
Degradation = enzyme levels drop → activity stops
🗑 Helps get rid of damaged or unneeded enzymes
✅ Keeps cell efficient and balanced
✨ Summary:
Unlike fast regulation (like allosteric or covalent changes), changing how much enzyme is made or destroyed is a slow, long-term strategy. It relies on controlling gene expression or protein breakdown.
ENZYME REGULATION SUMMARY
We have just discussed several different mechanisms that all can change the rate of an enzyme catalyzed reaction. These mechanisms work together to tightly control the enzymatic processes inside the cell. If one of these mechanisms becomes damaged, this can lead to alterations, that if uncorrected, can lead to a disease state. We will be exploring these themes in more detail with examples throughout the rest of the course.