hydrocarbons
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
what are hydrocarbons?
carbon and hydrogen bonded together
what are the naming conventions for the hydrocarbons?
based on the number of C atoms
meth-
eth-
prop-
but-
pent-
hex-
hept-
oct-
non-
dec-
bond types
single: -ane
double: -ene
triple: -yne
what are functional groups? what is the one we most commonly use?
a specific group of atoms or bonds within a molecule that dictates its chemical and physical properties and reactivity
we commonly use OH functional group (hydroxyl group)
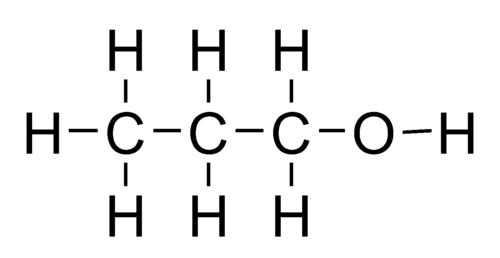
what is the name of this?
1-propanol
NOT 3-propanol, because you can rotate it so that the functional group would be on the left-most carbon
you want the smallest number possible
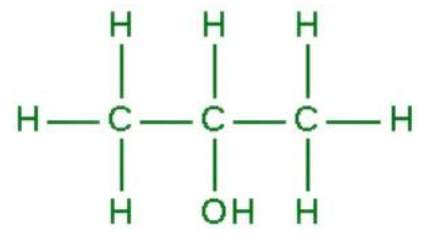
what is the name of this?
2-propanol
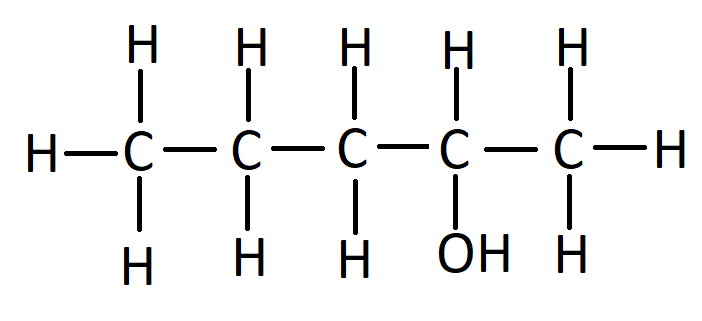
what is the name of this?
2-pentanol
NOT 4-pentanol
you want the smallest number possible
what are the effects of functional groups on melting/boiling points, etc.?
polar substances have higher boiling points than nonpolar substances because of more IMFs (dipole-dipole or hydrogen bonding)
stronger forces of attraction causes higher boiling points
larger electron cloud (more polarizable) means greater strength of attraction
larger surface area means more places to attract particles, meaning higher IMFs and therefore higher boiling points