UCM Biochem Chapter 6
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
binding energy
free energy released in formation of a large number of weak interactions between the enzyme and substrate
what does binding energy do?
established substrate specificity and increases catalytic efficiency
induced fit
modification of shape of an active site in an enzyme that occurs upon substrate binding
strategies to catalyze specific reactions
covalent, general acid-base, catalysis by approximation, metal ion
covalent catalysis
active site contains a reactive group that becomes temporarily covalently modified
Example of covalent catalysis
chymotrypsin
general acid-base catalysis
a molecule other than water plays a role of a proton donor or acceptor
Examples of general acid-base catalysis
histidine residues in chymotrypsin, carbonic anhydrase
catalysis by approximation
increases in the rate of a reaction that occur by bringing multiple substrates together along a single binding surface of an enzyme
Examples of catalysis by approximation
carbonic anhydrase binds carbon dioxide and water in adjacent site
metal ion catalysis
metal acts as an electrophilic catalyst by stabilizing a negative charge on the reaction intermediate, generates a nucleophile by increasing the activity of nearby molecules, or increases the binding energy of a enzyme-substrate interaction by binding to substrates
Examples of metal ion catalysis
zinc (2) helps form hydroxide in carbonic anhydrase, magnesium (2) stabilizes negative charge on rxn intermediate in EcoRV
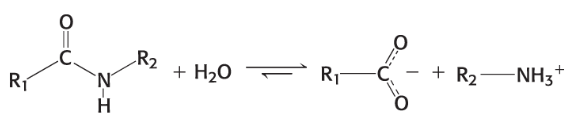
protease
cleave proteins by hydrolysis rxn (digest protein) and add a water molecule to peptide bond
chops protein into smaller things to be absorbed on carbonyl side
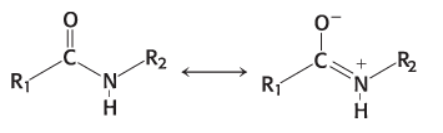
chemical nature of peptide bonds
kinetically stable due to resonance structures
thus they have double bond character
electrophilic (zap together)
tendency of a molecule to attract or acquire electrons
nucleophilic (bomb)
donate electrons or react at relatively electron- poor sites
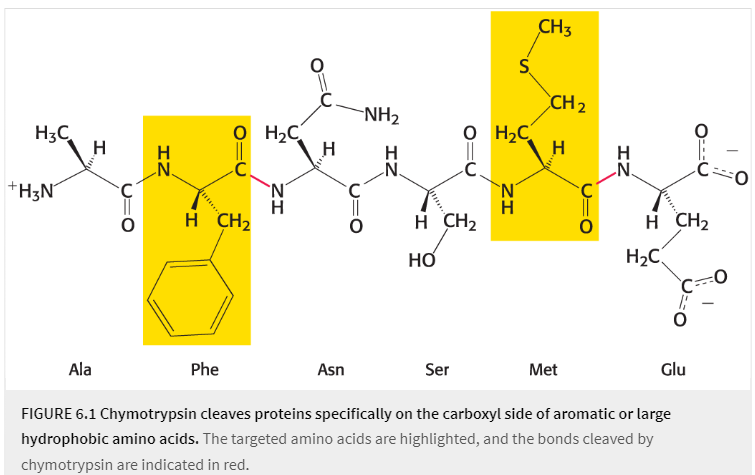
what enzyme cleaves peptide bonds selectively on the carboxyl-terminal side of a large hydrophobic AA (methionine or phenylalanine)?
good examples of covalent catalysis
serine 195
chymotrypsin
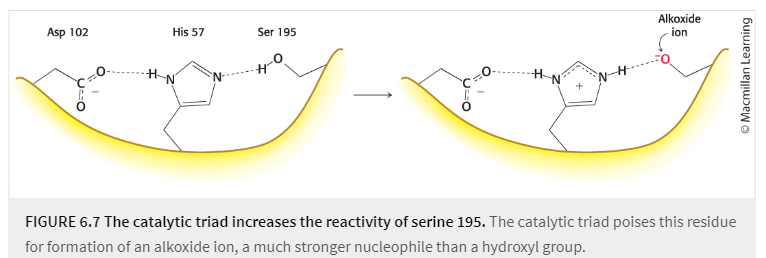
catalytic triad
Ser 195 sits in the cleft on the surface of chymotrypsin
a constellation of three residues, found in many proteolytic enzymes, in which two of the residues convert the remaining residue, usually a serine or cysteine residue, into the potent nucleophile
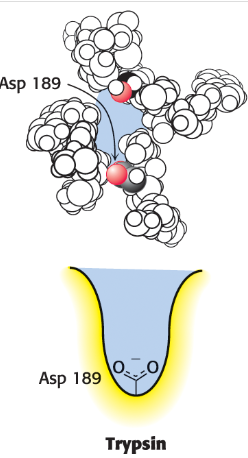
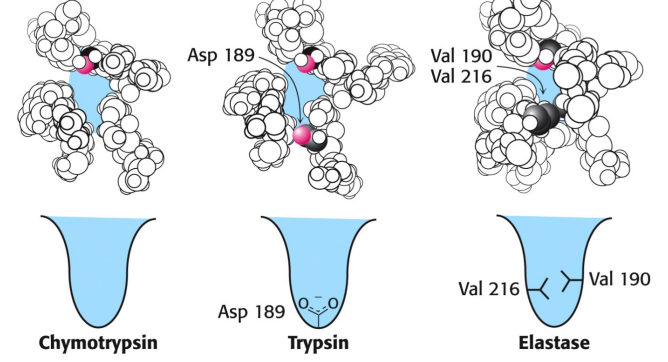
What does trypsin S1 pocket have that attracts and stabilizes (+) charged arginine or lysine residue in the substrate?
which helps with enzyme specificity
aspartate residue (Asp 189) at bottom of pocket
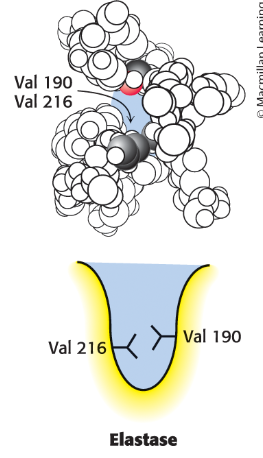
What does elastase S1 pocket have that close off the mouth of the pocket so only small side chains can enter (alanine and serine)?
bulkier valine residues (Val 190 and Val 216)
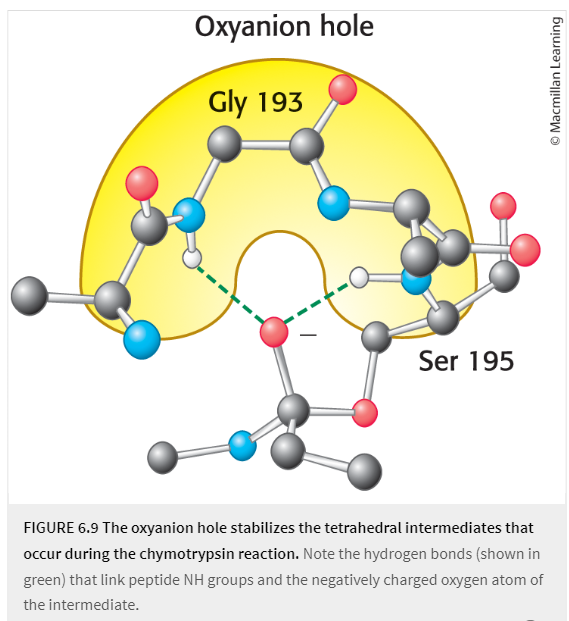
oxyanion hole
region on certain proteolytic enzxymes that stabilizes the oxyanion constituent of the tetrahedral intermediate of the rxn
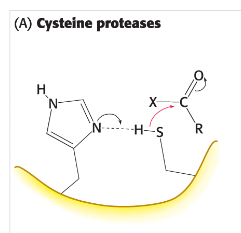
cysteine proteases
activated by the histidine to play role as nucleophile that attacks peptide bond. sulfur works as better nucleophile than serine
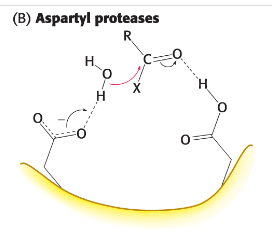
aspartyl protease
pair of aspartic acid residues act together to allow a water molecule to attack peptide bond. One Asp (deprotonated form) activates the attacking water by poising it for deprotonation. The other (protonated form) polarizes the peptide carbonyl group so it is susceptible to attack
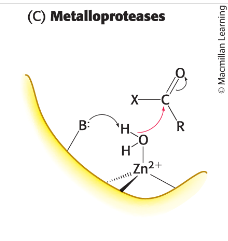
metalloprotease
metal ion that activates water molecule to act as nucleophile to attack peptide carbonyl group.
protease main job (steps)
activate water molecule or another nucleophile
polarize peptide carbonyl group
stabilize a tetrahedral intermediate
How is one way carbonic acid is formed?
carbon dioxide reacts with water to make bicarbonate ion and a proton thus forms H2CO3
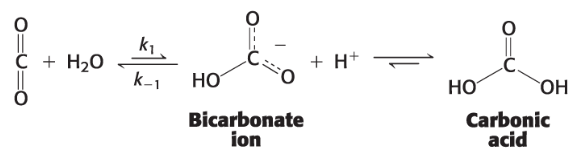
What is carbonic anhydrase?
that makes rxn much faster
enzyme that hydrates carbon dioxide to form bicarbonate HCO3-
What does carbonic anhydrease contain that aids in increasing chemical reactivity?
metal ions specifically zinc 2+
ligand
molecule or group that binds to receptor including metal ions
In carbonic anhydrase, zinc is typically bound to ligands, which is usually four or more molecules, such as?
three sites are nitrogen ligands from imidazole rings of three histidine residues and one water molecule
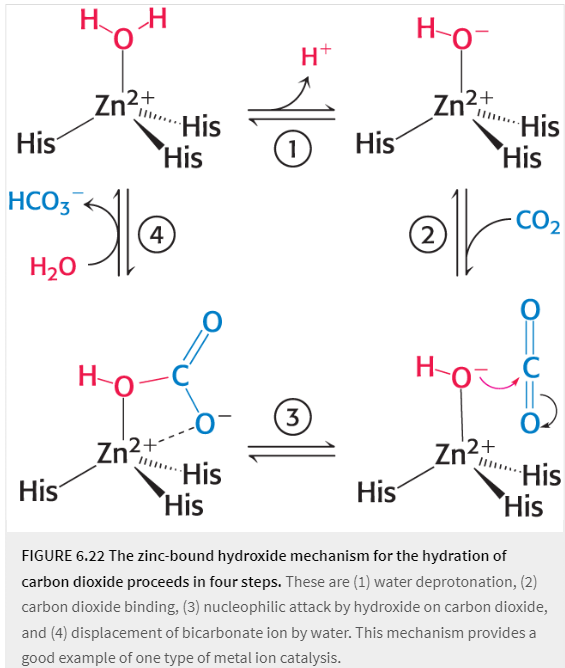
When pKa is lowered to 7 from the binding of zinc and carbonic anhydrase, zinc attacks (nucleophile) carbon dioxide better than water
zinc ion facilitates the release of the proton from the water molecule which generates hydroxide ion
CO2 substrate bind to enzyme’s active site and is positioned to react with hydroxide ion
hydroxide ion attacks the CO2 , converting it into bicarbonate ion, HCO3-
catalytic site is regenerated with the release of HCO3- and the binding of another water molecule
The rate of CO2 hydration by carbonic anhydrase increases with the concentration of what?
A buffer (1,2-Dimethlybenzimidazole)
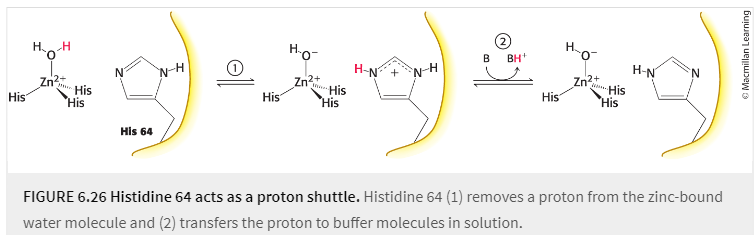
proton shuttle
group that facilitates the transfer of protons in or out of the active site of an enzyme
Carbonic anhydrase 2 has evolved a proton shuttle to allow buffer components to participate in the rxn from the solution. What is the primary component of this shuttle?
histidine 64
Four characteristics of enzymes
biomolecules that catalyze chemical rxns
composed of proteins and RNA
many require coenzymes and/or metal ions
accelerate chemical rxns by providing an alternate pathway for rxn to occur
Important unique features of enzymes
tremendous catalytic power (a lot of product and quick)
specificity (specific products formed)
regulation (enzymatic activity is finely controlled → turned on and off when needed)
enzyme specificity
each enzyme catalyzes a specific rxn on sm. range of substrates
Explain enzyme active sites (Enzyme increase rate of rxn in three major ways:)
lower Ea by stabilizing the transition state
provide an alternate path for product formation
reduce entropy by orienting the substrates appropriately for rxn to occur
change environment
binding of substrate releases H2O
transition state model
an enzyme’s active site is designed to bind to TSǂ stronger than either the product or the reactant. The active site promotes the formation of the TSǂ
Four methods of enzyme catalysis
covalent
general acid-base
metal ion
proximity and orientation (approximation)
covalent catalysis
Enzymes can form a weak covalent bond to a substrate molecule, which can stabilize a reaction intermediate
A water molecule or second substrate then attacks the covalent intermediate
AA are usually used a nucleophiles
Coenzymes are usually electrophiles
general acid-base catalysis
Donation of a H+ in a reaction by the enzyme or substrate and not a free H+ in solution
AA side chains, N-Terminus and C-Terminus may be used
place base next to H2O to make good hydroxide
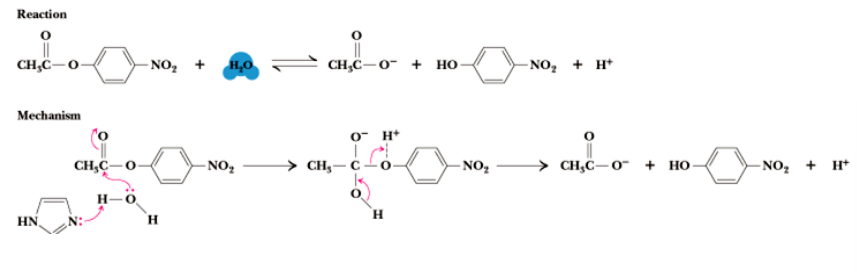
metal ion catalysis
Electrophile - stabilizes/or induces a negative charge on a substrate or intermediate
May alter the acidity of a functional group creating a strong nucleophile
May aid in substrate binding
Electron transfer reactions
add metal ion to make more electrophilic (partial (+) or polar)
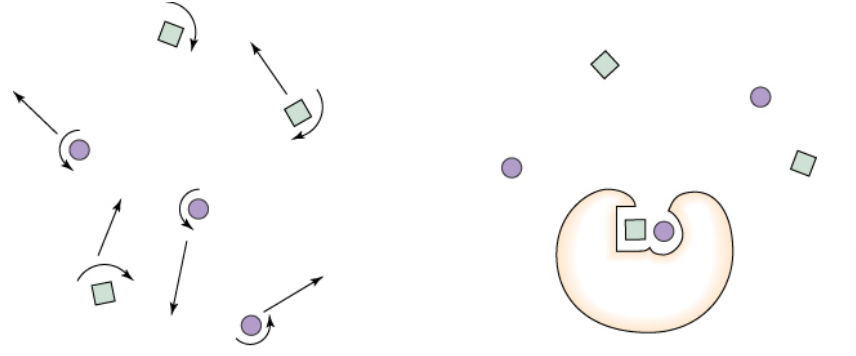
proximity and orientation
Reactions occur when a fruitful collision between reactants happen
Enzymes bring the reactive species in close contact (increase the chance of collision)
Enzymes place reactive groups in the correct orientation
make sure rxn groups are allined
specificity of serine protease
determined by a binding pocket for substrates side chain
pocket wouldnt form without disulfide bonds
serine protease catalytic triad
Ser 195 acts as nucleophile
His 57 acts as a general acid/base
Asp 102 stabilizes and positions
His57 in correct orientation