Internal energies: Physical Chemistry
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Internal Energy
The sum of all kinetic and potential energies in the system.
For a system of many particles, we cannot know the absolute internal energy. Only changes in internal energy matter.
Work and Heat’s effect on internal energies
Work done on a system and heat added to the system increase the internal energy of the system.
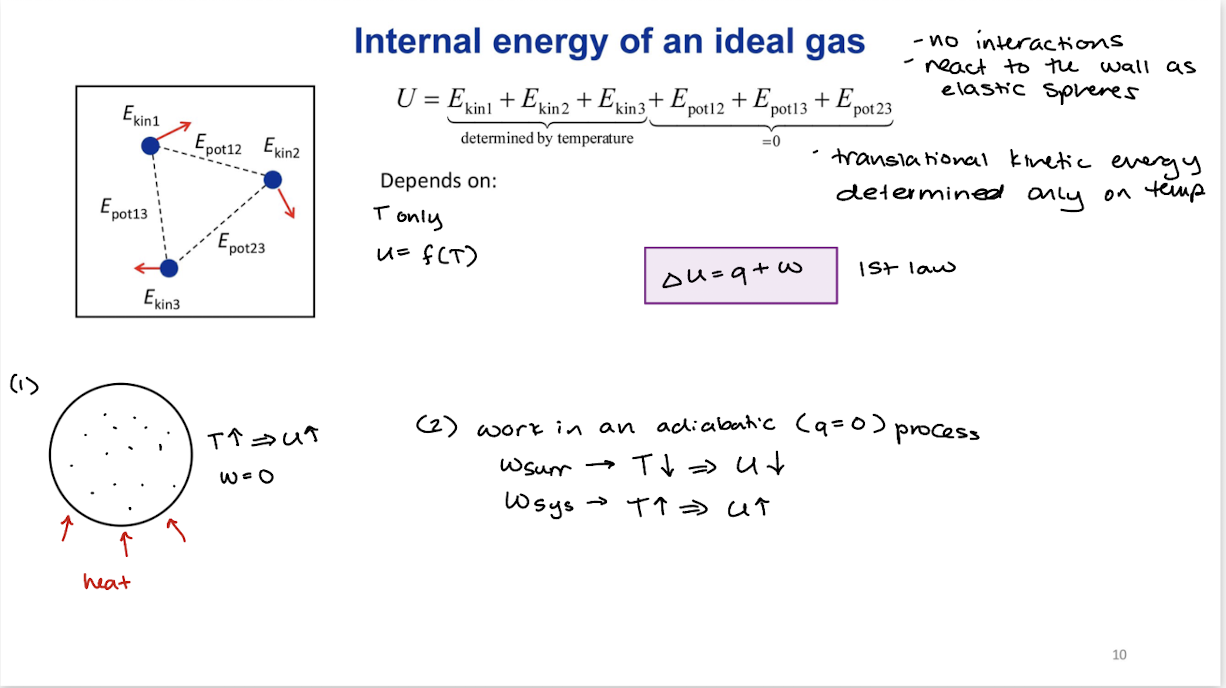
Internal energy of an ideal gas
∆U=q+w= f(T)
Depends on temperature only
Temperature effects the kinetic energy of internal molecules
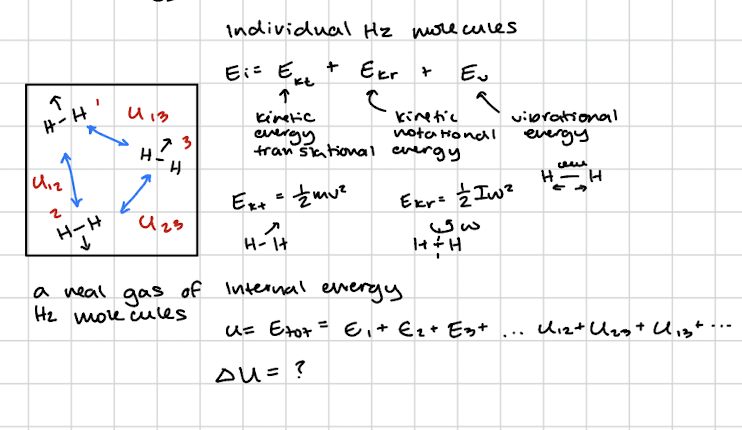
Individual H2 molecules
Function of Internal Energy
Internal Energy is a STATE function
∆U=q+w
Q and W are both path functions but ∆U is a state function
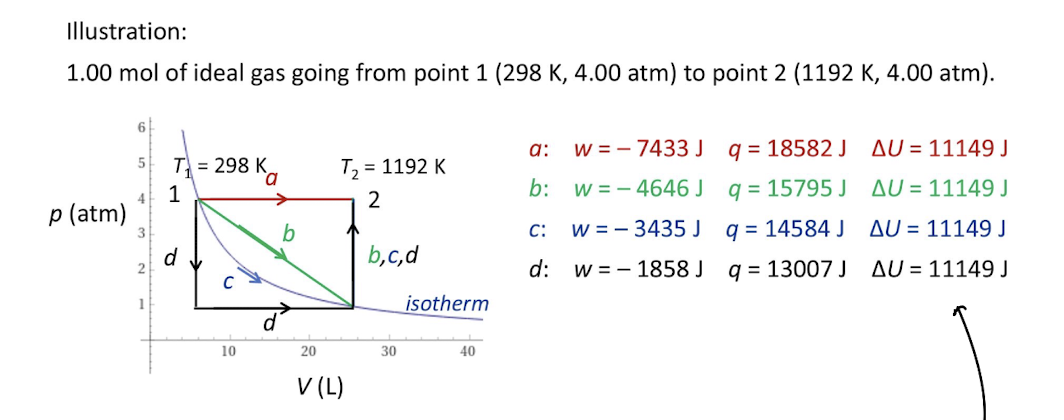
Internal energy vs temperature
Consider a process with constant volume (isochoric process ∆V=0)
∆U=qv (heat added at constant V)
qv=Cv∆T=nCmv∆T
∆U=nCv∆T
can be used for other T→T processes once found out because ∆U is a state function
Isochoric
Constant volume
First Law of Thermodymaics
First take a look at a process that occurs without heat transfer (adiabatic process)
∆U=q+w (q=0) → ∆U=w
The change of internal energy of a system in an adiabatic process equals to the work put into the system.
Wsurr=-Wsys=-∆U An open cylinder getting colder releasing gas
Adiabatic expansion: w<0 and ∆U≤0: the system does work on the surroundings at the expense of its internal energy.
An isolated system (no work or heat transfer)
w=0 q=0 ∆U=0
The internal energy of an isolated system is constant.
We cannot extract work from the system, leaving its internal energy unchanged.
Organisms require an external supply of energy to do work as they live.
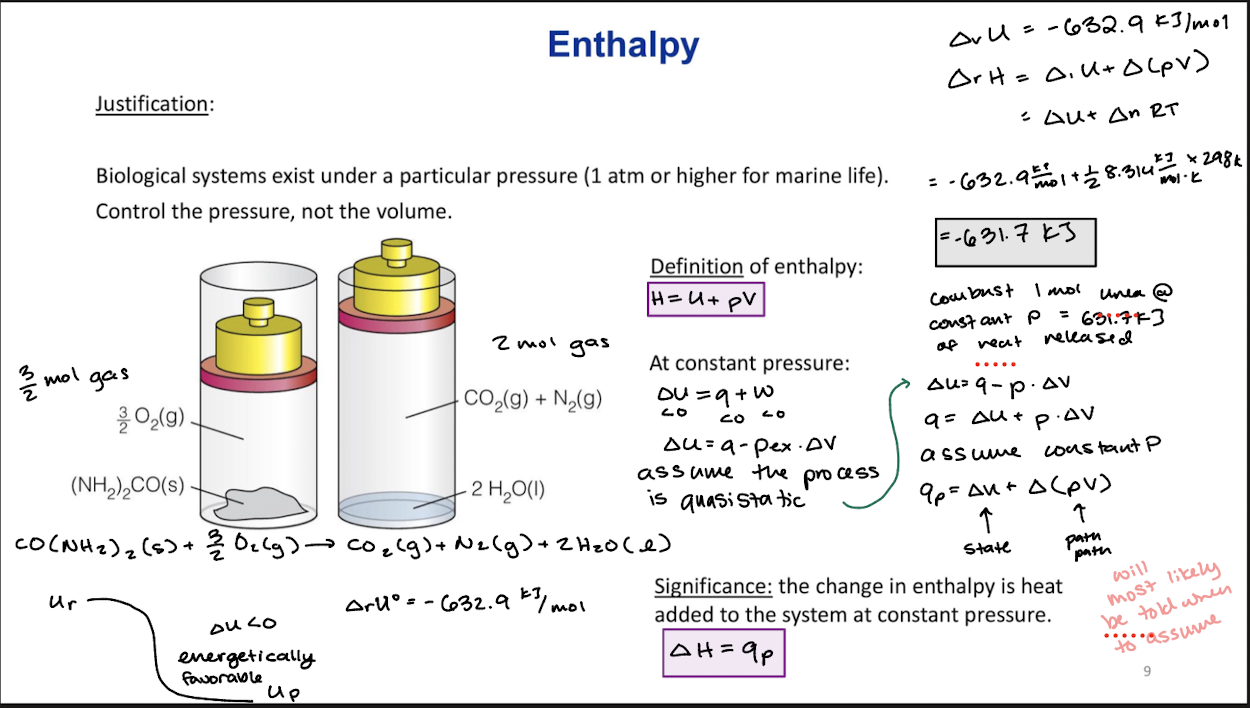
Enthalpy
Justification: Biological systems exist under a particular pressure (1 atm or higher for marine life). Control the pressure, not the volume.
H=U+pV
at constant pressure
∆U=q+w
qp=∆U+∆(pV)
Significance: The change in enthalpy is heat added to the system at constant pressure.
∆H=qp