C6 - prep of carbon dioxide , hydrogen oxygen
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
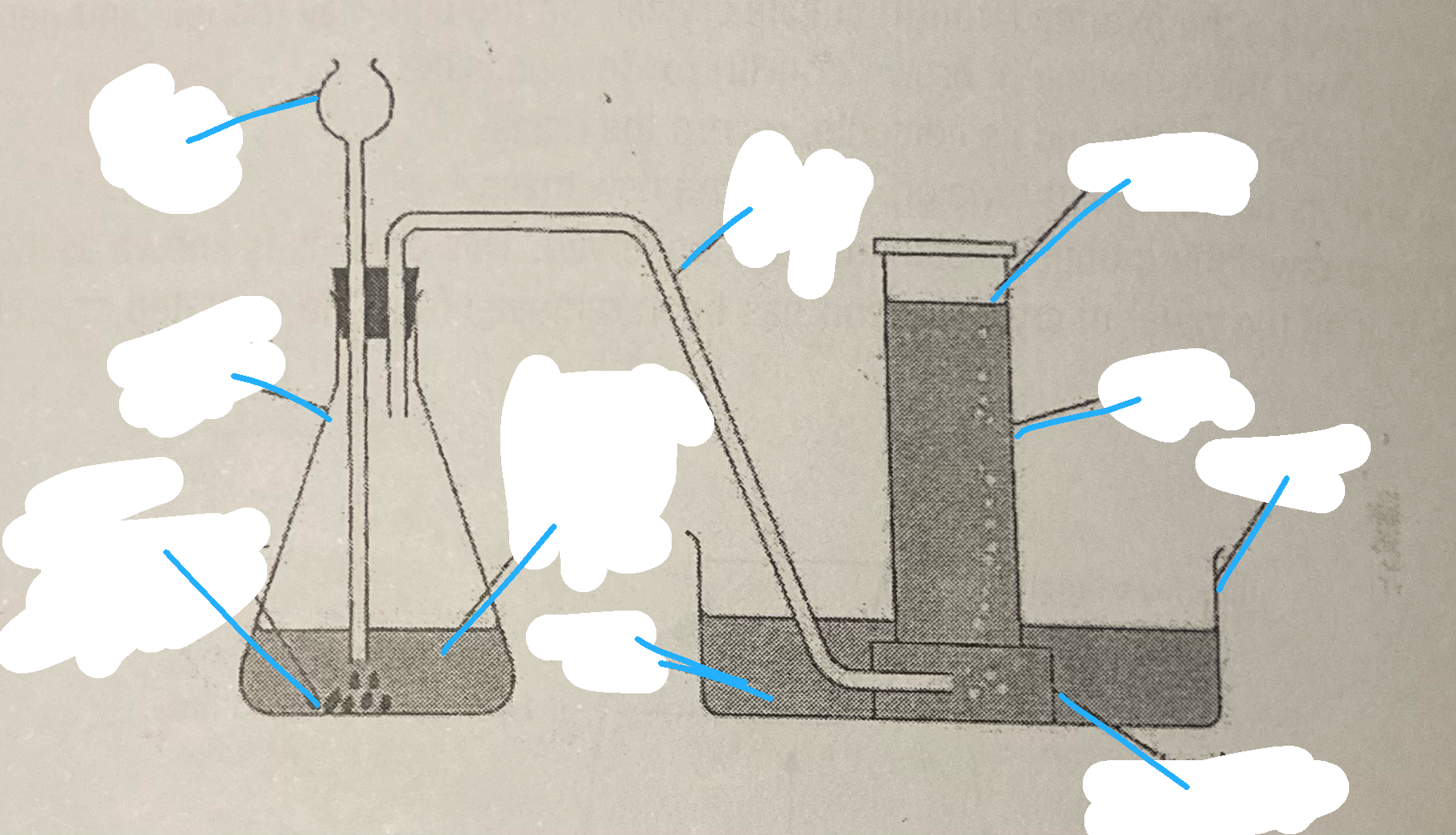
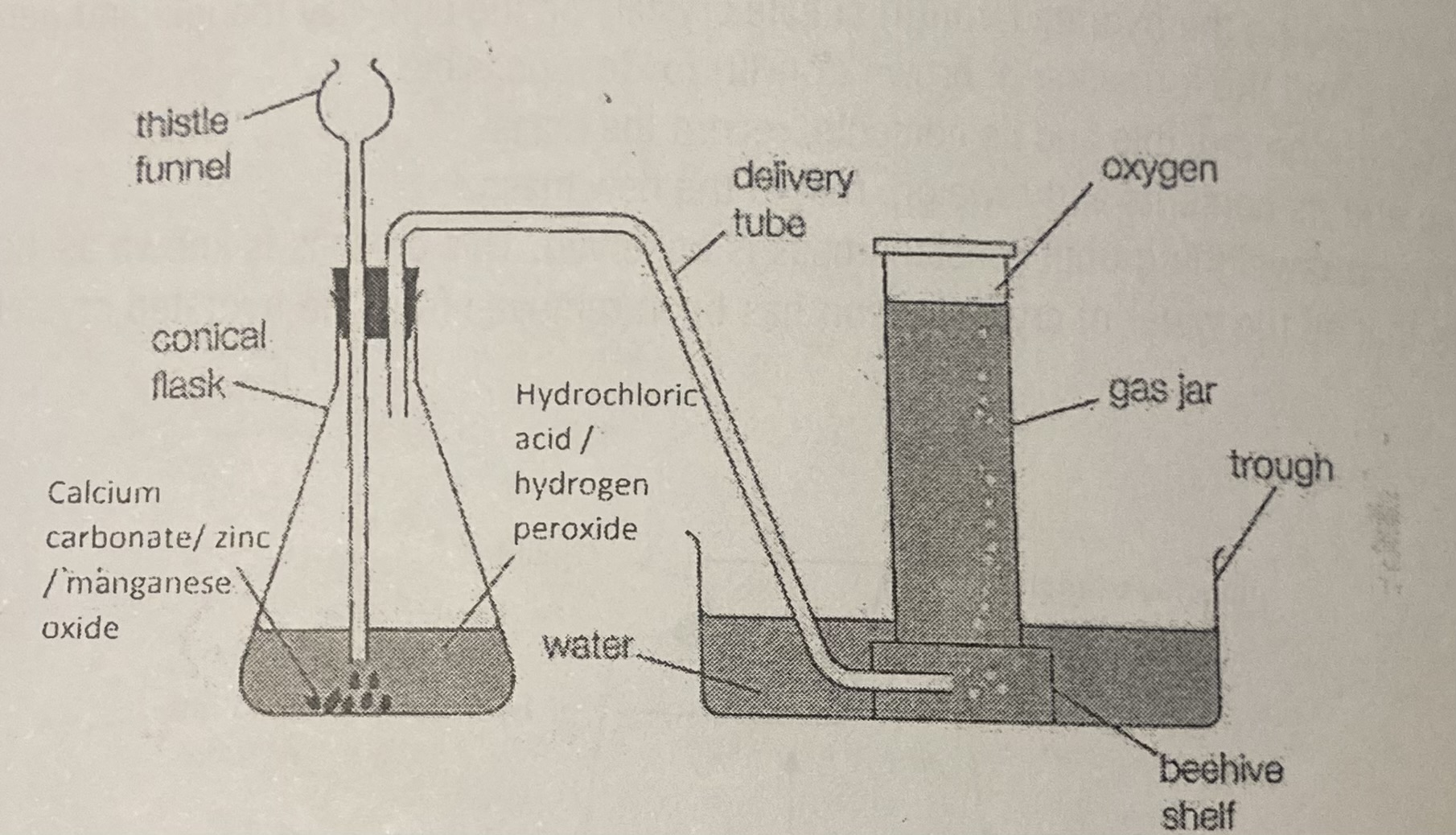
prep of carbon dioxide
Carefully place the calcium carbonate into the conical flask.
Collect 50 cm3 of hydrochloric acid in a small beaker.
Connect the delivery tube and thistle funnel to the conical flask with the delivery tube in a basin of water.
Have three gas jars/test tubes filled with water also inverted in the basin of water.
Carefully pour the hydrochloric acid into the thistle funnel, wait 5 s then carefully place the first water filled gas jar/test tube over the end of the delivery tube. Allow the gas jar/test tube to fill with gas (when all of the water has been displaced).
Leave the gas jar/test tube inverted in the water to retain the gas.
Repeat step 5 until three gas jars/test tubes of gas have been collected.
Remove each gas jars/test tube from the water as required for the following tests.
test for carbon dioxide
To the first test tube insert a lit splint – flame is extinguished
To the second test tube add 1 cm3 of limewater, stopper and shake – turns milk or cloudy white
record your observations To the third gas jar/test tube add 5 drops of universal indicator, stopper and shake . Add a little deionised water if no change observed and shake again. Acidic so colour will change to be yellow or orange
prep for hydrogen
The same method is used as for carbon dioxide except zinc (or sometimes magnesium ribbon) is used in place of calcium carbonate. Collect 3 gas jars/test tubes of hydrogen gas in the same way.
testing the gas
lit splint squeaky pop
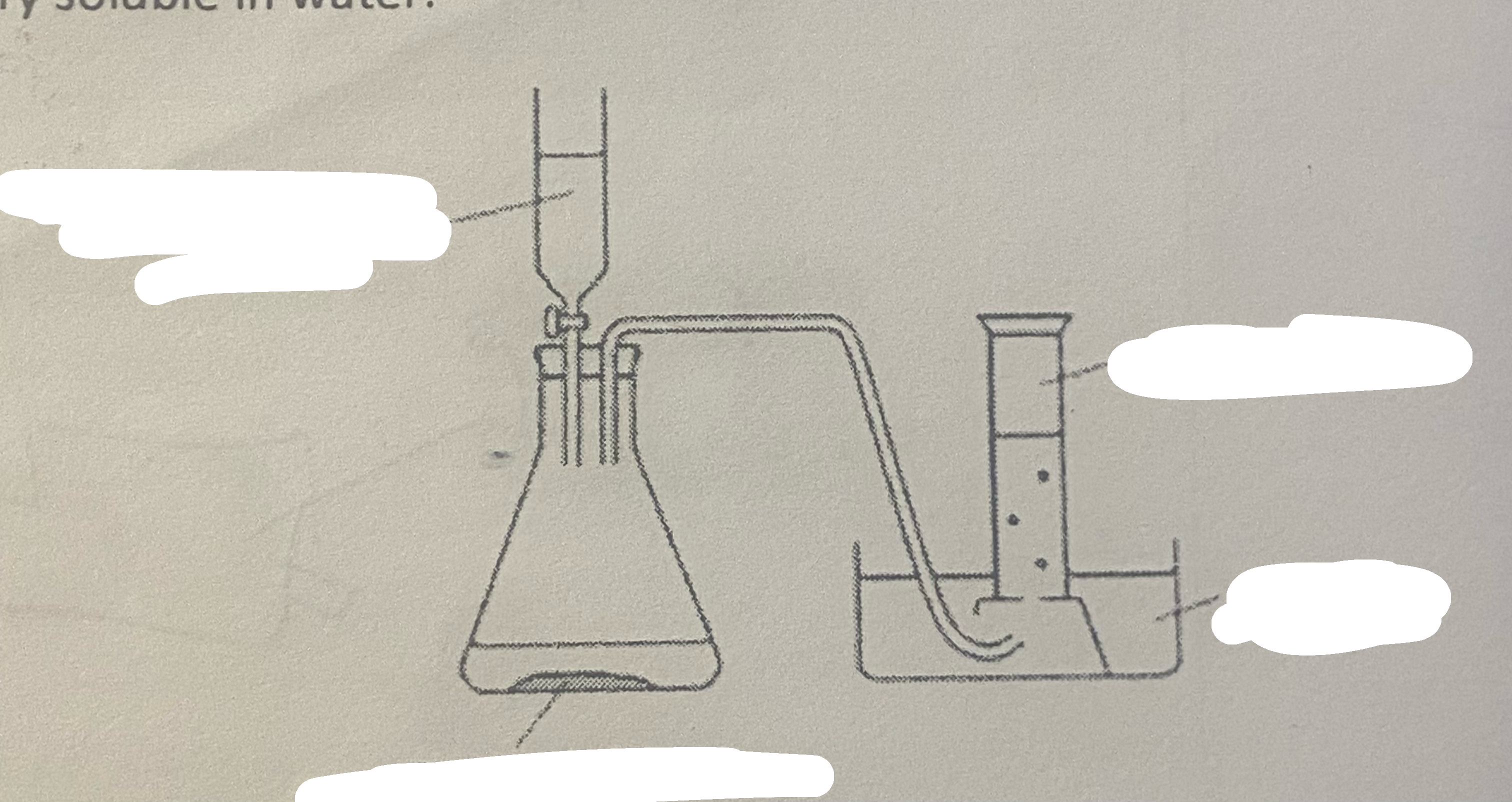
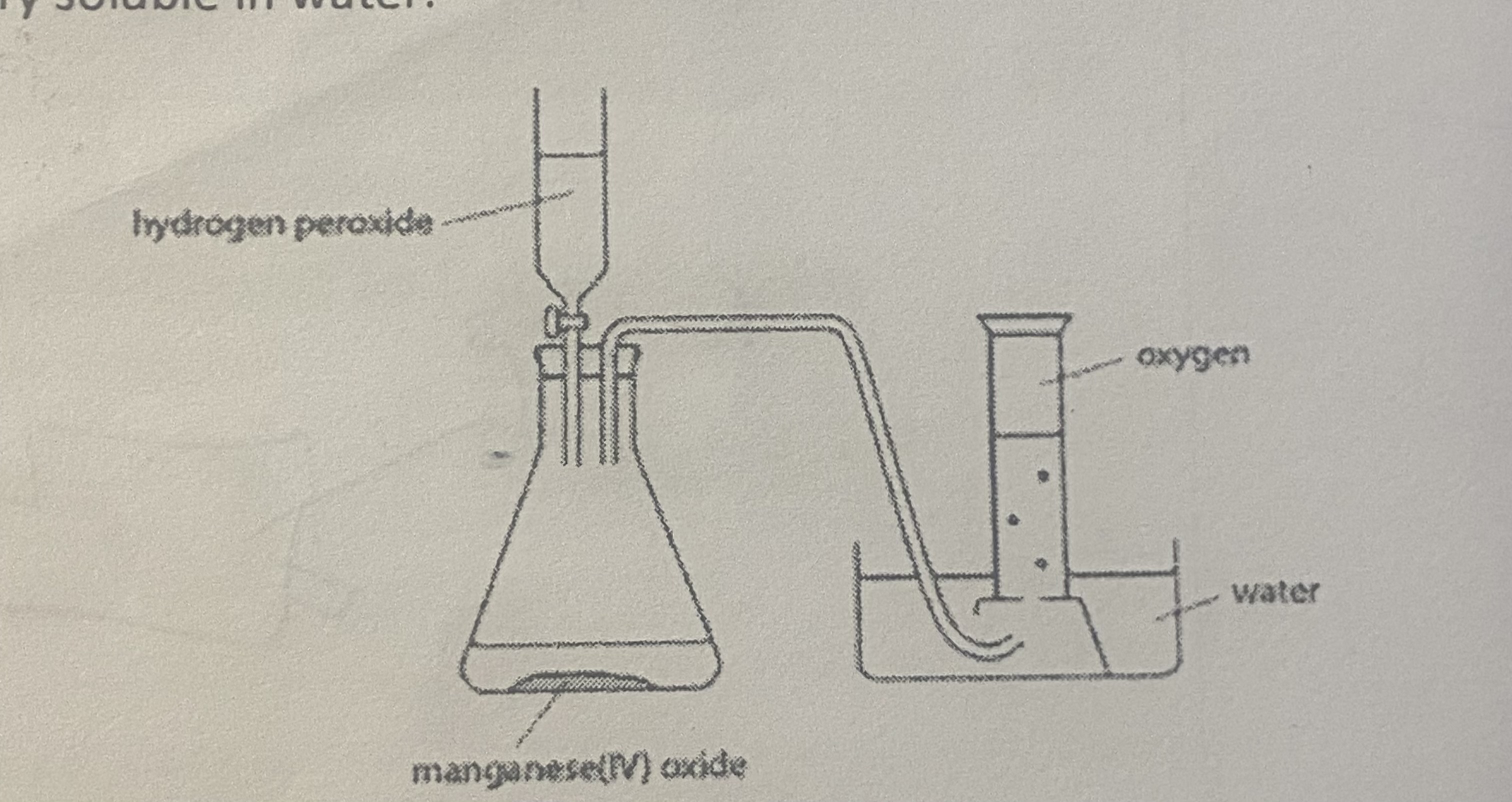
prep for oxygen
same but with manganese (IV) oxide and 50cm3 of hydrogen peroxide
test for oxygen
glowing splint relights