enzyme regulation and inhibition
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
allosteric
activity is changed by change of 3d structure brought about by small molecules like substrates or regulators
allosteric enzymes qualities
quaternary structure
cooperativity between subunits
larger than other enzymes
cooperativity of subunits
binding by a substrate affects binding at all other subunits - becomes more active
how is rate versus [S] plot affected due to subunit cooperativity
sigmoidal
not hyperbolic
at low [S] affinity is low, but more turn into high affinity
activation and inhibition of allosteric enzymes
an allosteric enzyme has active and inactive forms
allosteric activator can stabilise the active form (R)
allosteric inhibitor will stabilise the inactive form (T)
PFK-1
enzyme which catalyses fructose-6-phosphate → fructose-1,6-bisphosphate
neg allosterically modulated by phosphoenolpyurvate and atp
shows sigmoidal curve
hwo do allosteric regulators affect Km and Vmax
affect Km
activators will have lower Km (higher affinity)
inhibitors will have higher Km (lower affinity)
no Vmax
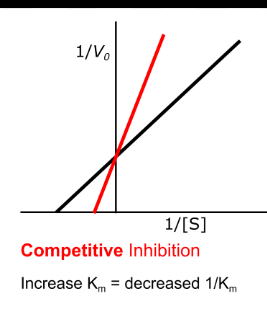
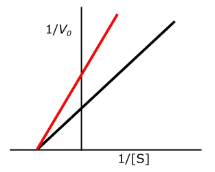
effect of constants with competitive inhibition
km is increased
will reach the same Vmax
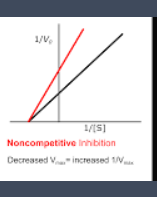
effect of non competitve inhibition on constants
vmax is decreased
more s does not restore the rate
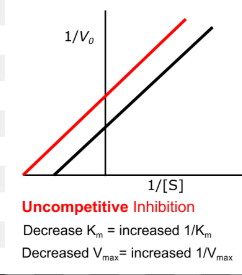
effect of uncompetitive inhibition on constants
both vmax and km is decreased
binds to enzyme-substrate complex
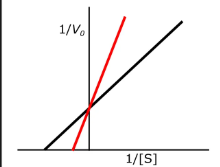
which inhibition is this?
competitive
which inhibition is this?
non competitive
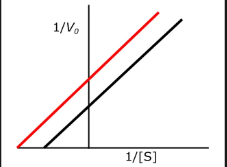
which inhibition is this
uncompetitive