CSUF CHEM 100 Midterm 3 Study Guide
1/81
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
Acidic or Basic: Ph = 1
Acidic
Acidic or Basic: Ph = 12
Basic
What is a Ph of 7
neutral
What does a strong acid/base do?
Completely ionizes in a solution
What does a weak acid/base do?
Partially ionizes in a solution
Arrhenius Theory of ACIDS
H+ ions are produced in a solution
Arrhenius Theory of BASES
OH- ions are produced in a solution
What are the 2 sources for acid rain
Sulfur dioxide from power plants (burning coal)
and
Nitrogen oxides from combusion
Fossil Fuels
Fuels formed from decayed organic matter over millions of years
How is crude oil refined?
Fractional Distillation
heated in a furnace
becomes a vapor and enters the distillation column
separated into different fractions according to BOILING POINT
vapor condenses to a liquid
Do hydrocarbons with more carbon atoms have lower boiling points?
No
Oxidation or Reduction Reaction:
Na^+ + e- —→ Na
Reduction - because e- is on the left
Oxidation or Reduction Reaction:
C + 2O²- —→ CO2 + 4e-
Oxidation - because e- is on the right
Which side of the fuel cell does oxidation happen?
anode
Which way do electrons flow in a fuel cell?
form the anode to the cathode
List the advantages of a fuel cell:
H2O is released
Cradle-to-Cradle
plans for a second use of the original product
recycling (cans/bottles)
Cradle to Grave
consumer uses product then disposes of it (no second use intended)
goes to landfill or incinerator
Which type of polymer is more easily recycled?
Thermoplastic because they can be heated to a melting point and can form other products using molds.
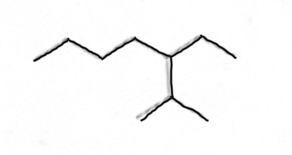
Is this hydrophobic or Hydrophilic
Hydrophobic
Benefits of using GMOs
better crop yield
increased vitamin content in foods
crops become resistant to pests/herbicides
Saturated Fatty acids
Bad for your health
single bonds
solids at room temp
Unsaturated fatty acids
Better for your health
double bonds
liquids at room temp
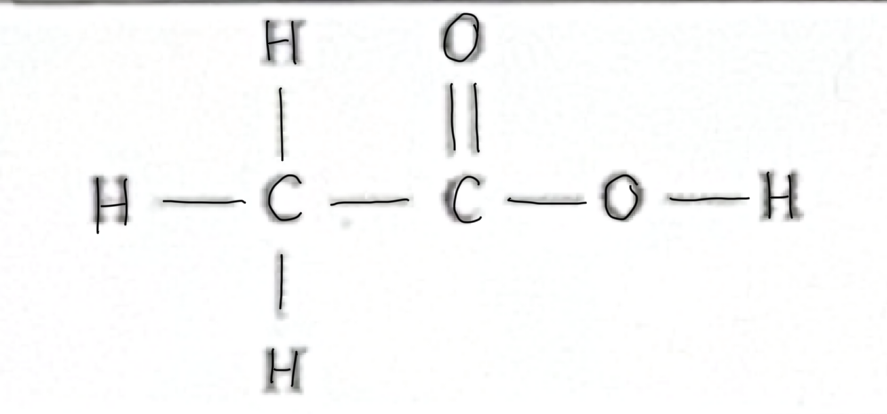
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
carboxylic Acid
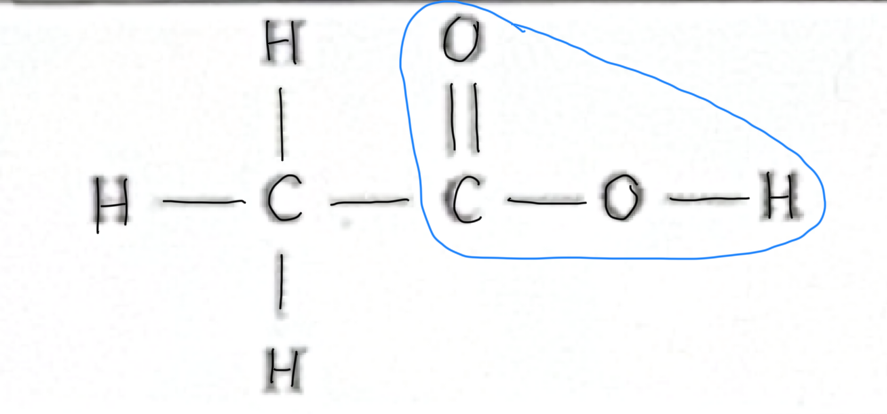
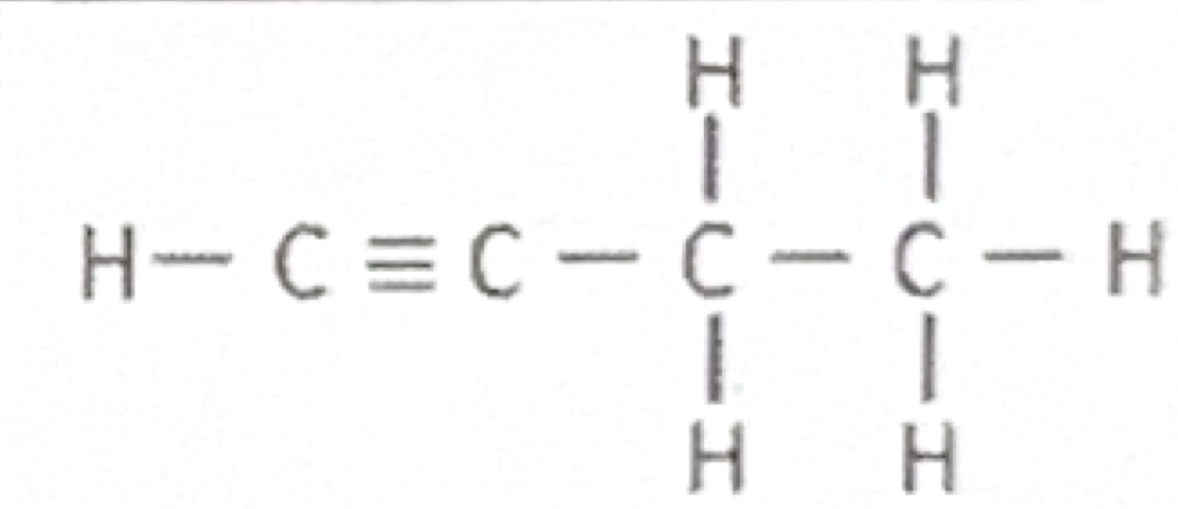
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
Alkyne
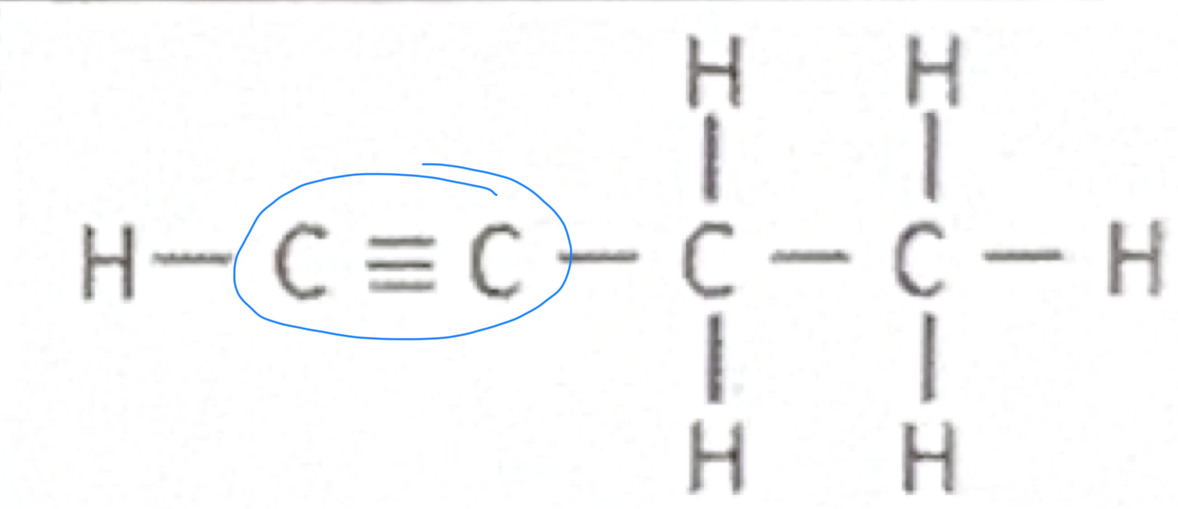
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
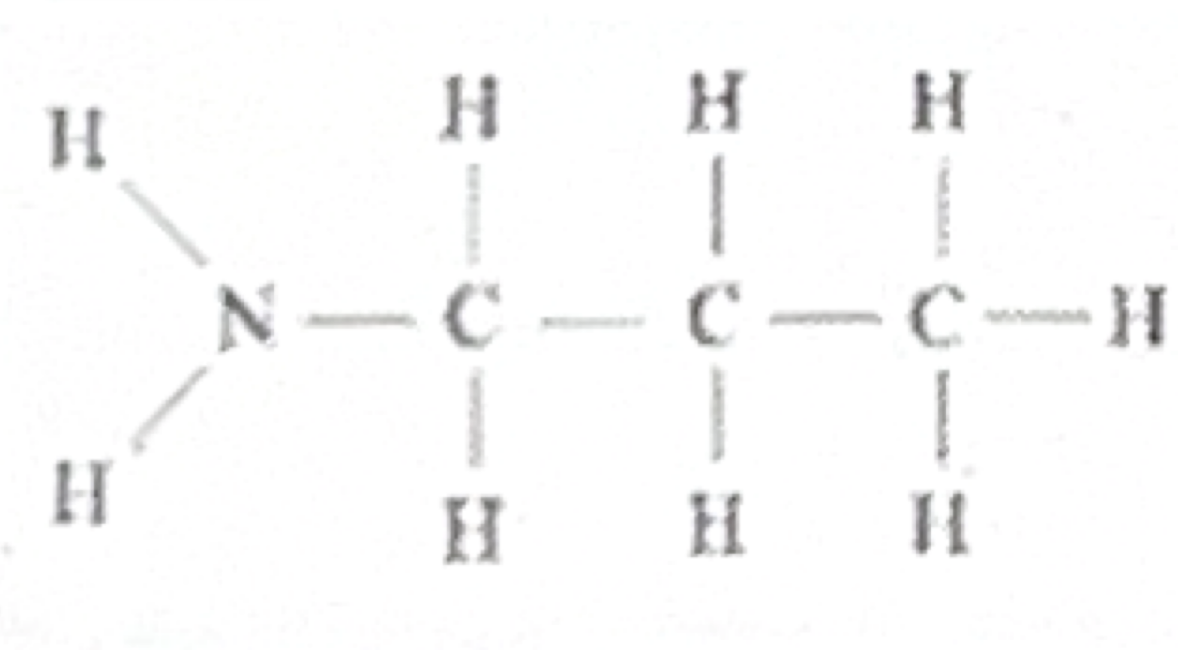
Amine
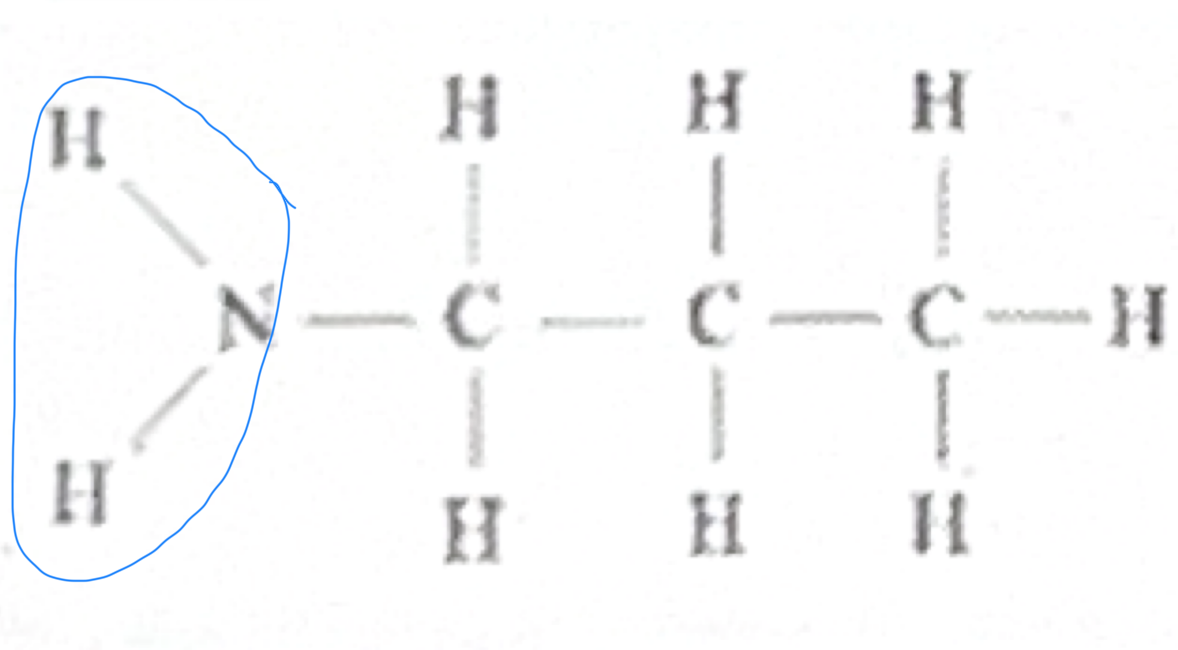
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
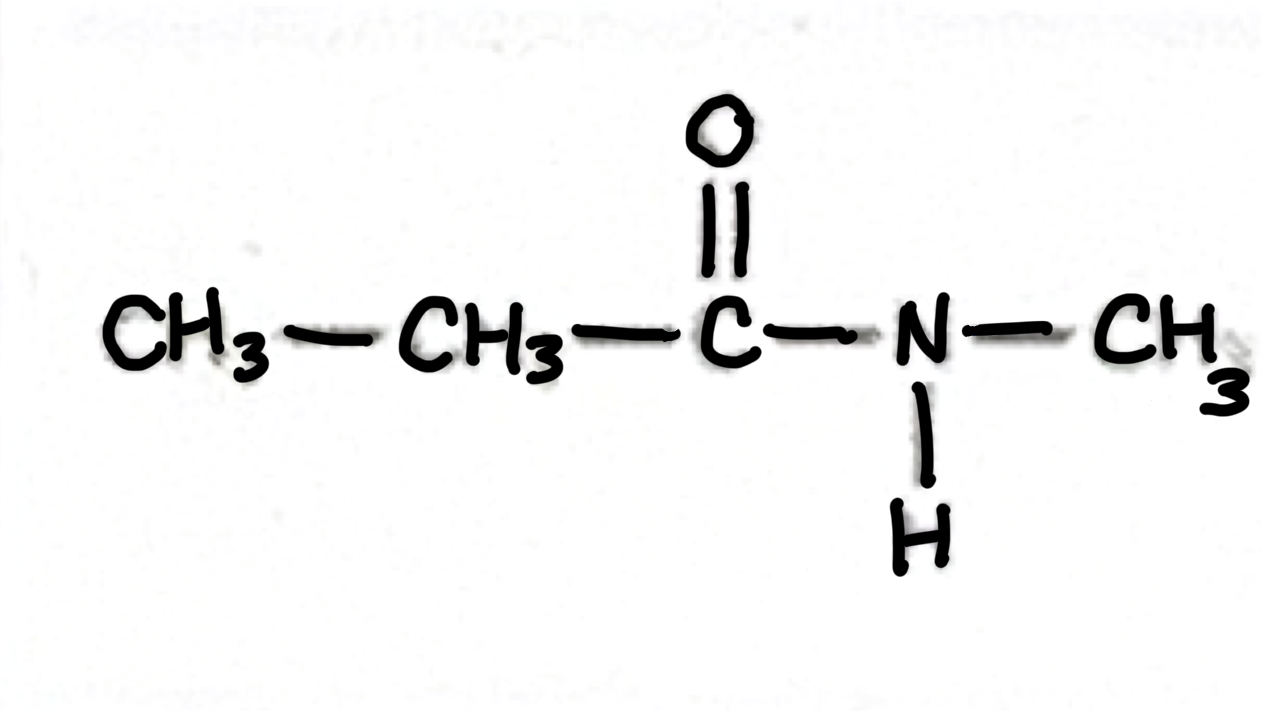
Amide
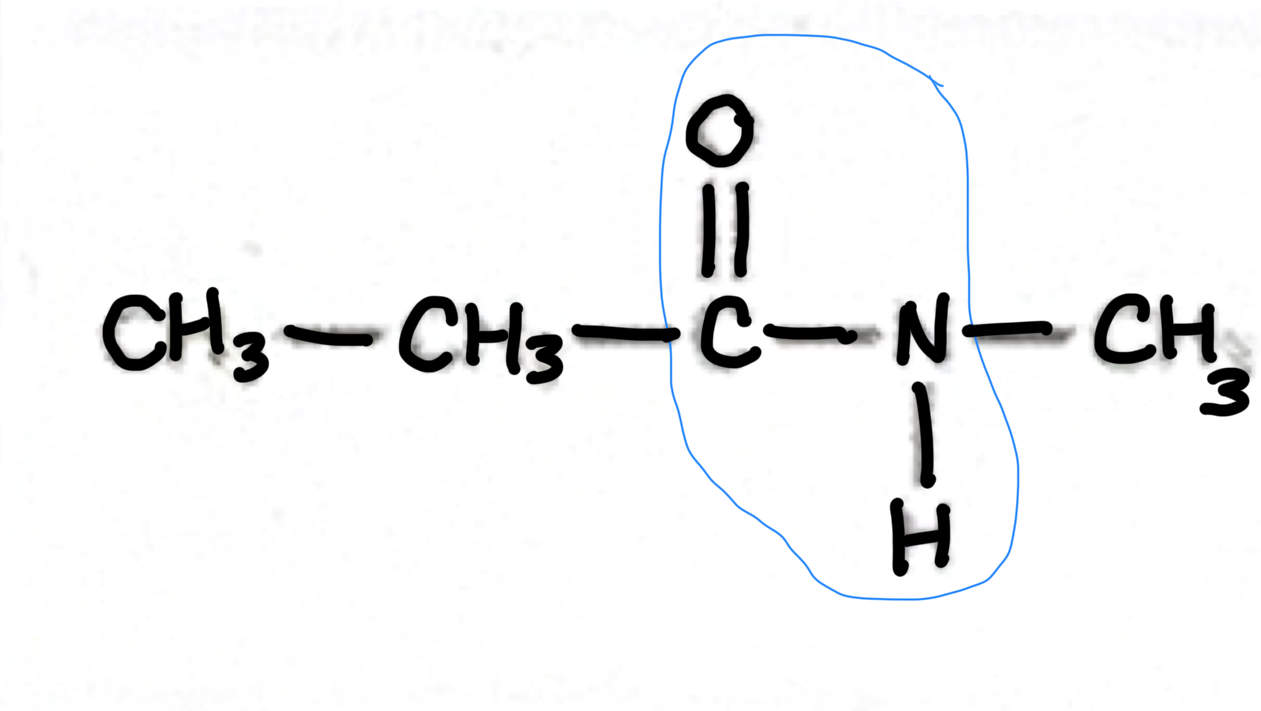
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
Alkane
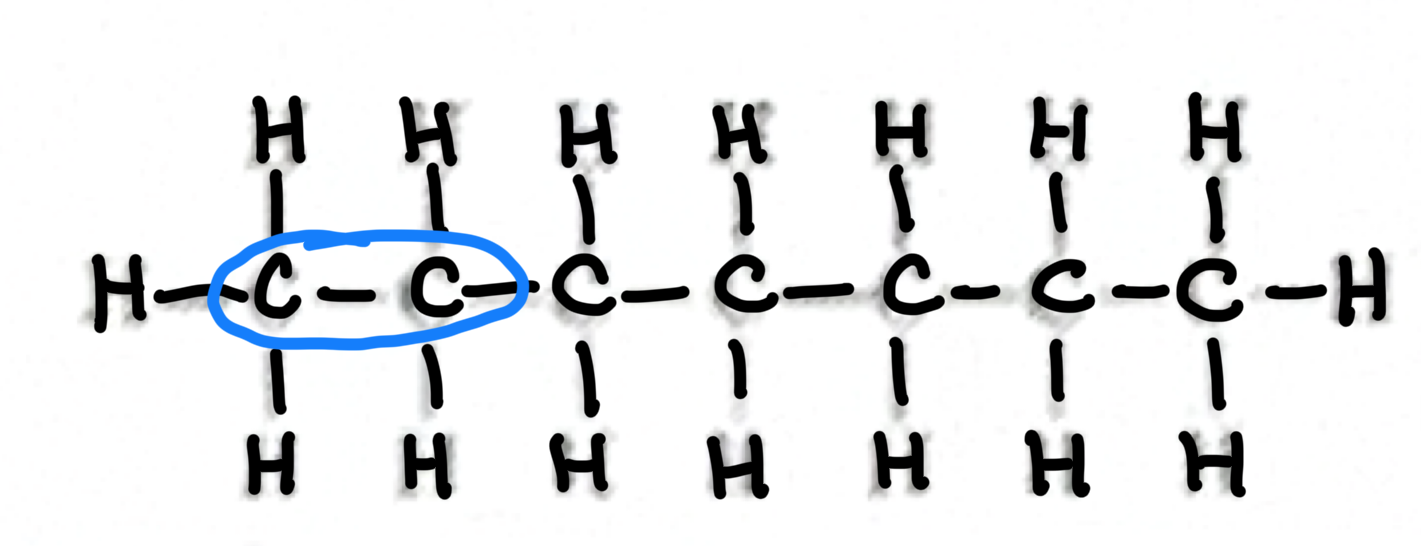
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
Aldehyde
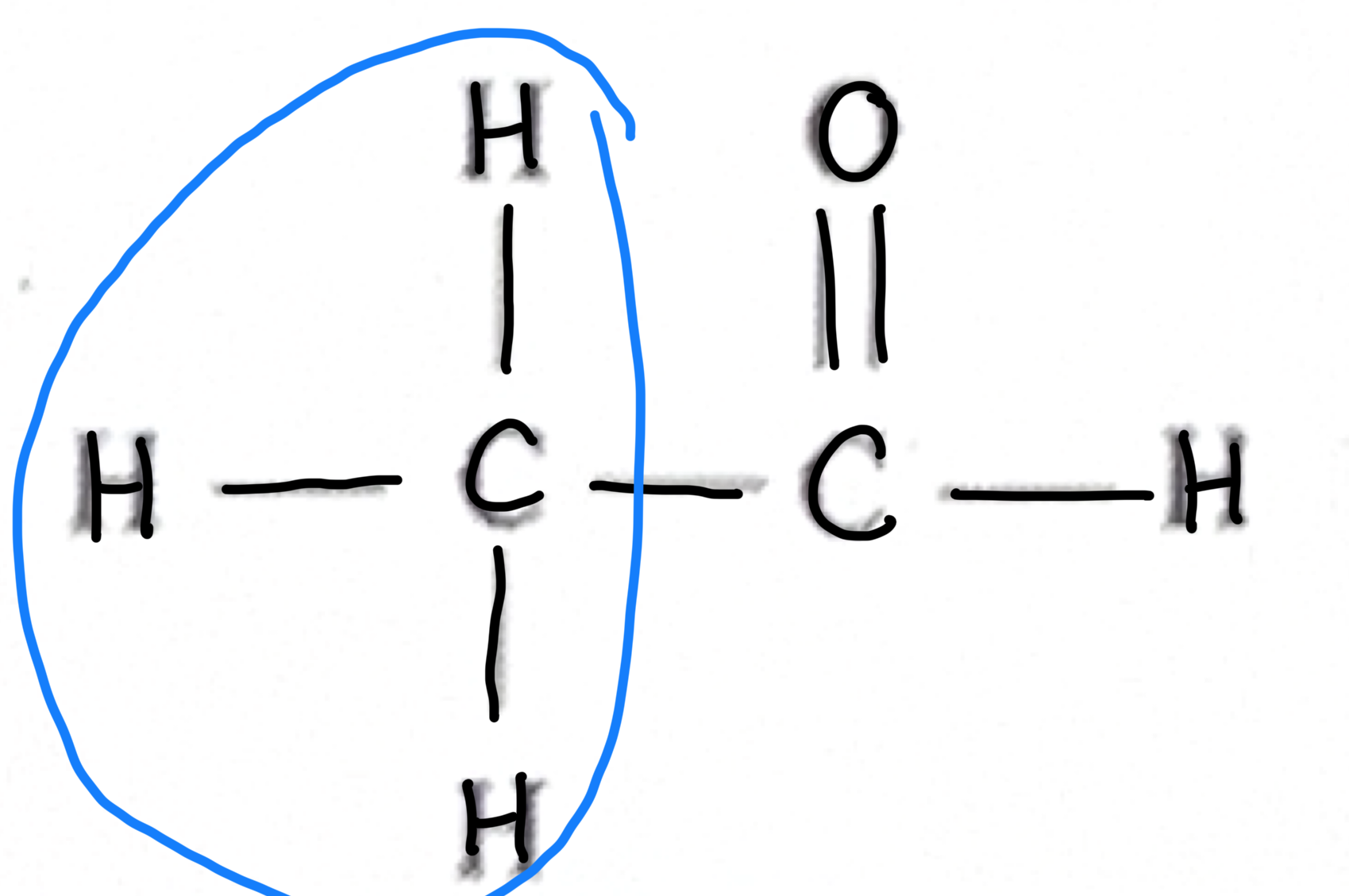
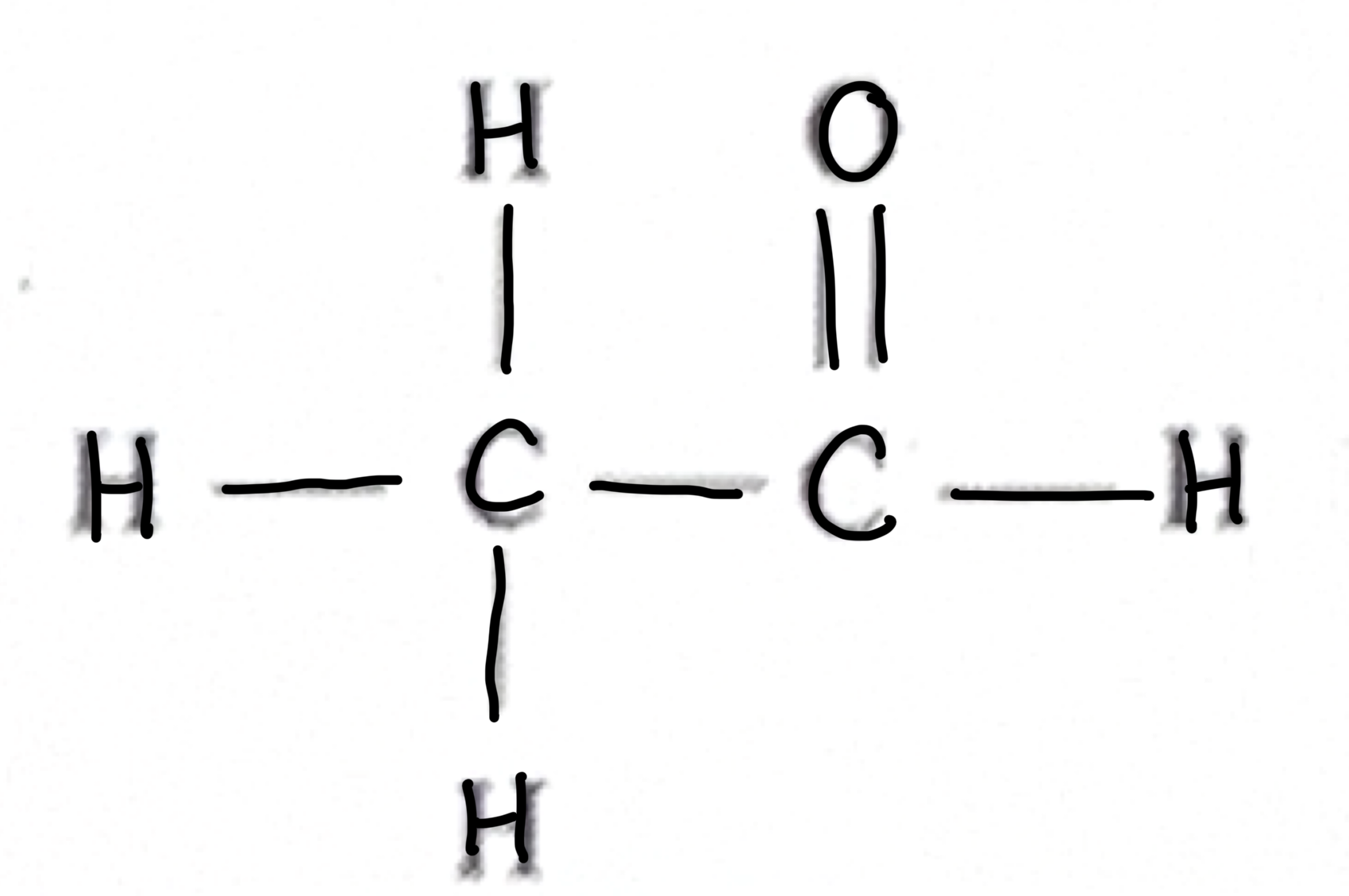
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
Alkene
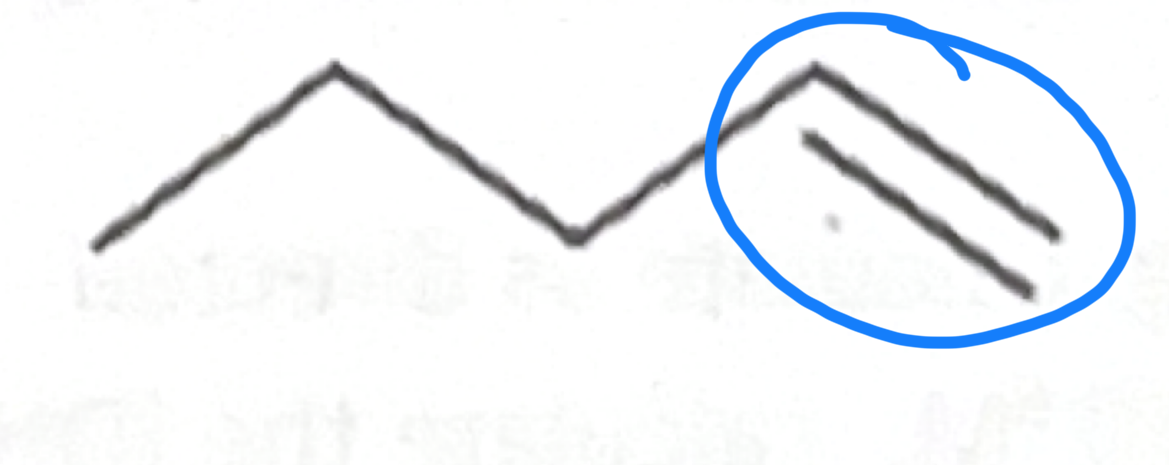
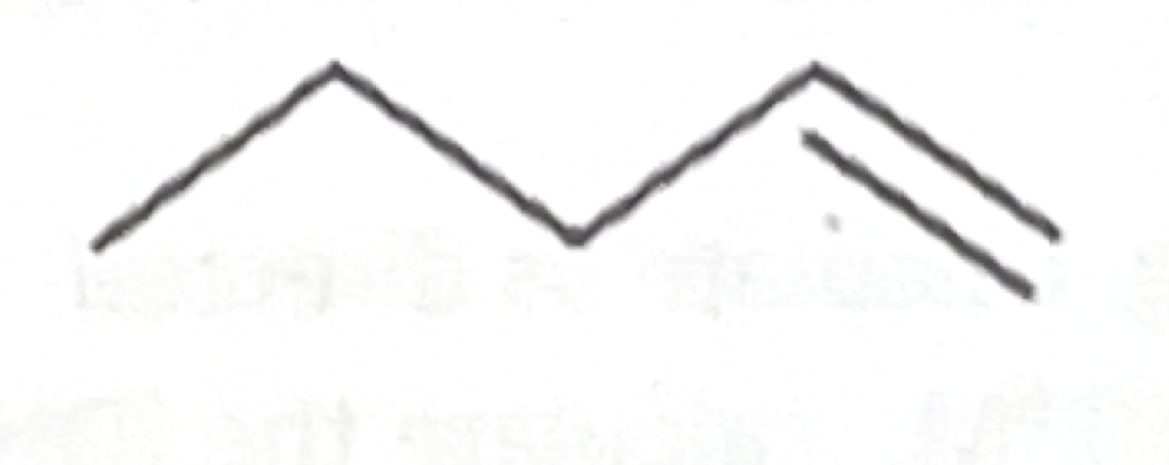
Which functional group does this belong to?
carboxylic acid
Alkyne
Amine
Amide
Alkane
Alkene
Aldehyde
Ketone
Ketone
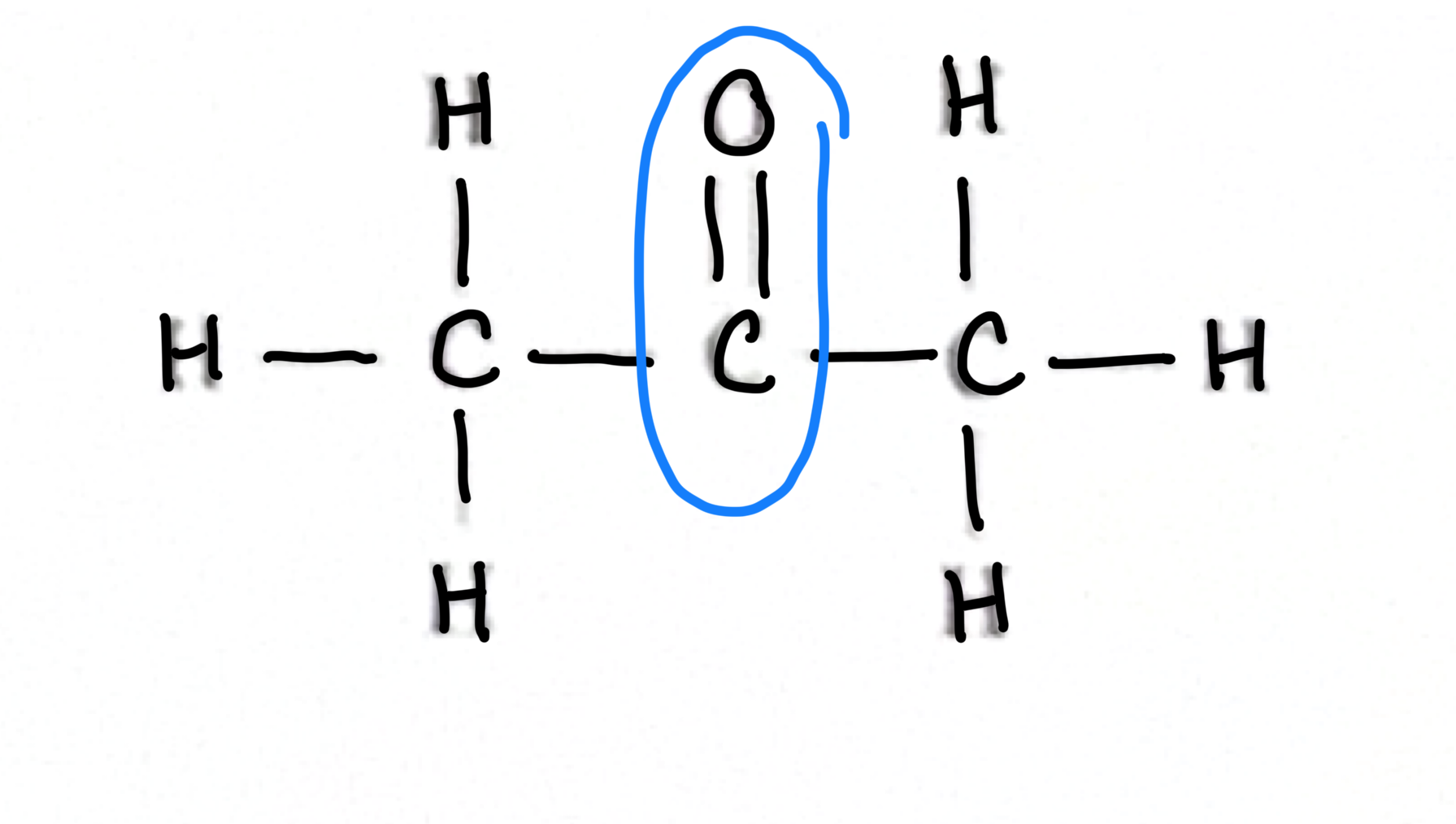
which has the least distance between atoms
triple bonds
which has the most bond energy
triple bonds
What do hydrocarbons contain
only C & H
What is a heteroatom
any atom that is NOT: C or H
Acedic Ph
< 7
Basic Ph
> 7
Normal Rain Ph
5.6
What does the Clean Air Act do?
regulate emissions
Example of Fossil Fuels
Coal, Oil, Natural Gas
Combusion Produces:
CO2,, H20, and Energy
Major Greenhouse gasses
CO2, CH4, N2O, water vapor
What happens in an oxidation reaction
electrons are lost
What happens in a reduction reaction
electrons are gained
What is the great pacific garbage patch
a rotating current trapping plastic waste
What are plastics?
long chain polymers made from monomers
Which resin code is most easily disposed of
#1
Nutrients
Substances needed for life
Macronutrients
carbs, fats, proteins
Micronutrients
vitamins/minerals
amino acids
building blocks of proteins
biomolecules
biological molecules
complete proteins
have all essential amino acids
Monosaccharides
glucose fructose
Disaccharides
sucrose lactose
polysaccharide
starch cellulose
simple sugars
mono + di
complex carbs
polysaccharides
Whole grains
includes the bran, germ, and endosperm
refined grains
only contains the endosperm
How does digestion work?
food is put into the mouth
salive starts breaking it down
moves to the stomach
then the intestine
makes glucose
What are lipids
mostly nonpolar
hydrophobic
aromatic
benzene rings
properties of acids
conduct electricity in solution, are corrosive, and react with metals to produce hydrogen gas and with bases to form a salt and water
properties of bases
bitter-tasting, feel slippery, and turn red litmus paper blue. They have a pH greater than 7
What is acid rain?
acids that have been dissolved in water
energy
the ability to apply a force over a distance
power
is the amount of energy generated or used in a certain amount of time
watt
number of joules of energy transferred per second
First Law of Thermodynamics
states that energy cannot be created or destroyed
What is released during the combustion process?
energy, CO2, and H2O
What is an electrochemical?
a chemical reaction that involves the transfer of electrons, either generating an electric current or using electricity to drive a chemical change
What is Electrosis?
direct electric current (DC) to drive a non-spontaneous chemical reaction,
What is a fuel cell?
device that can produce power from certain chemical reactions
What is the definition of life cycle assessment?
a systematic method to evaluate the environmental impacts of a product, process, or service throughout its entire life cycle, from raw material extraction to disposal
lightweighting
strategically reducing the mass of an object or component while maintaining or improving its required mechanical properties
what type of polymers are more easily biodegradable and why?
aliphatic polyesters and polyamides
Kevlar polymer
Strong
crystallite
small, distinct, and ordered region within a solid material where atoms, ions, or molecules are arranged in a regular, repeating crystal lattice structure
Hydrophobic
hates water
Hydrophilic
Water loving
Make Plastic Cups
crude oil obtained
Refine that crude oil
Drilling and refining require energy
Petroleum byproducts used to make plastic need to be transported to a factory to make the cups
All of the energy is accounted for in these steps
The cups are being made
Packaging and raw materials
Transported to a distribution center
Consumer purchases
Recycled or landfill or incinerate