lecture 4: drug-receptor theory, antagonists
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
the majority of drugs are clinically useful as…
antagonists
chemical antagonism
substances combine in solution so that the effects of the drugs is lost i.e. the agonist is chemically altered by the antagonism
give an example of chemical antagonism
inactivation of heavy metal ions is reduced with the addition of a chelating agent (e.g. dimercaprol). (the heavy metals can interact with transporters or enzymes, often blocking or disrupting their normal functions)
pharmacokinetic antagonism
(change in drug metabolism) reduction in the amount of drug by another drug via absorption, distribution, metabolism or excretion
give an example of pharmacokinetic antagonism
for patients taking warfarin (anti-coagulant and thins blood to reduce risk of heart attack and stroke) have to be careful when treated with some antibiotics as they may stimulate the metabolism of warfarin, reducing its effective concentration in the bloodstream
physiological antagonism
the interaction of 2 drugs with opposing actions in the body (both drugs will be agonists)
give an example of physiological antagonism
noradrenaline raises arterial blood pressure by acting on the near beta-1 receptors and peripheral blood vessels, alpha-1 receptors, whilst histamine (H1) lowers arterial pressure by causing vasodilation
histamine acting through H2 receptors increases acid secretion in the gut, while omeprazole (treats acid reflux and ulcers in the stomach and intestines by reducing the amount of acid produced) counteracts this by inhibiting the proton pump
non-competitive antagonism
blocks some steps in the process between receptor activation and response (i.e. does not compete with the agonist for the receptor site)
give an example of non-competitive antagonism
verapamil and nifedipine inhibits L-type calcium channels to cause relaxation of smooth muscle and lowers blood pressure
ketamine inhibits NMDA (ligand-gated) channels by physically blocking the channel pore
competitive antagonists
molecules that bind to the receptor in the same place as an agonist but following the binding, no response is initiated
very high concentrations of antagonists and/ or antagonists with very high affinity to a receptor compared to the agonist will lead to the receptor most likely to be occupied by antagonists, unless…
the agonist is in excess
the concentration-response curve shifts right with…
increasing antagonist concentration (no change in maximum height) because the antagonist is reducing the potency of the agonist
what happens to the conc-response curve when irreversible antagonists block enough receptors
the curve will shift down (reducing Emax)
dose ratio
how many more times is the agonist needed in the presence of an antagonist (measure of the shift, normally at the EC50)
dose ratio =
concentration of agonist in the presence of antagonist/ concentration of agonist in absence of antagonist
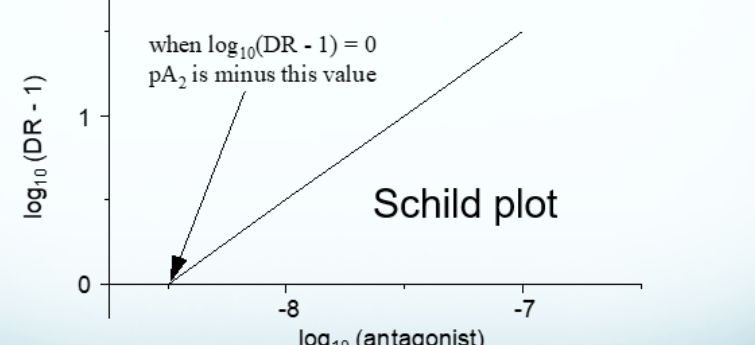
schild plot
shows the relationship between the dose ratio and the antagonist
how can we measure the affinity of the antagonist
by measuring the dose-ratio in the conc-response curve for an agonist caused by the addition of increasing concentrations of a competitive antagonist
schild equation
dose ratio - [Xb]/Kd + 1
Xb is the concentration of antagonist
Kd is the antagonist affinity constant
when log10(DR-1) = 0, PA2 is…
minus that value
PA2 =
-log10(Kd) or -log10(molecular concentration of antagonist that gives a dose ratio of 2)
when dose ratio is 0, 50% of receptors are…
occupied
when full agonists are added in the presence of partial agonists, why does the conc-response curve shift right?
because the full agonists’ potency is loss (increase in EC50)
give an example of an irreversible antagonist
aspirin
how can you tell if something works irreversibly
if you wash away the first antagonists and add agonists, it will produce a reduced maximum response