IB Chemistry HL - Oxidation and Reduction Reactions
0.0(0)
Card Sorting
1/13
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
1
New cards
oxidation
gaining oxygen or forming a bond with oxygen
2
New cards
oxidizing agents
K2Cr2O7, K2MnO4
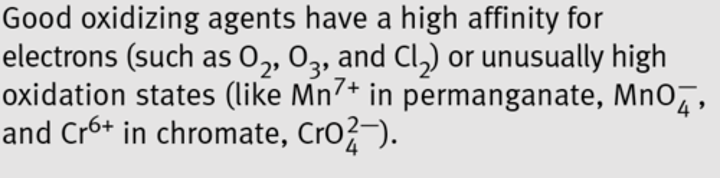
3
New cards
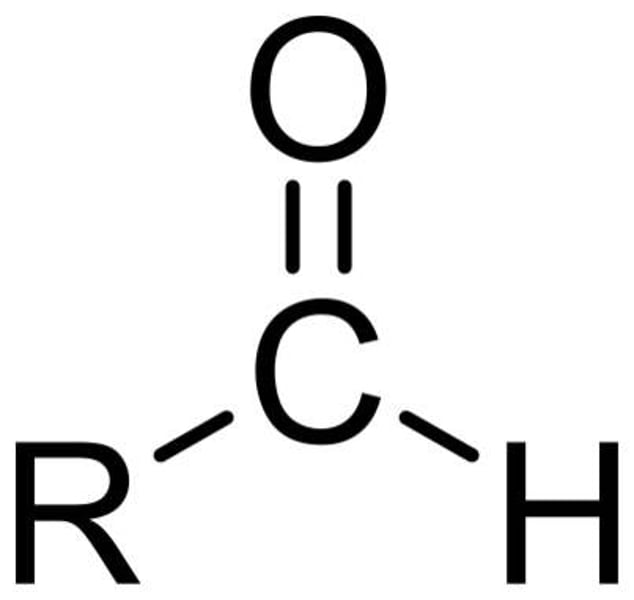
oxidation of a primary alcohol with distillation
aldehyde
4
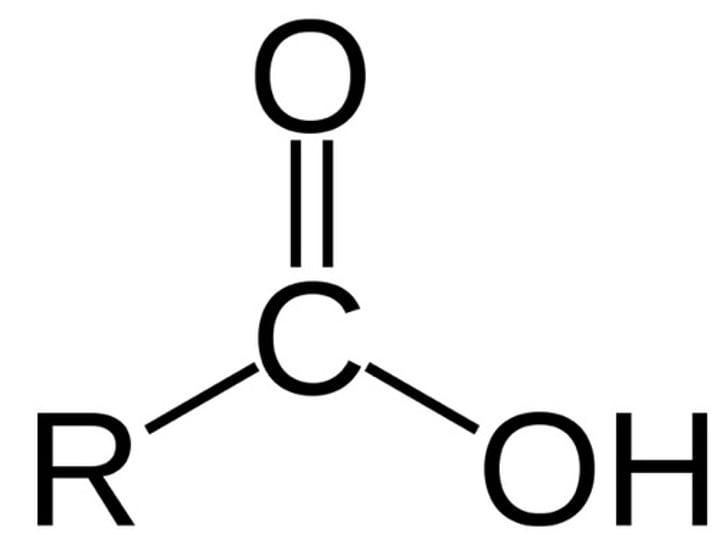
New cards
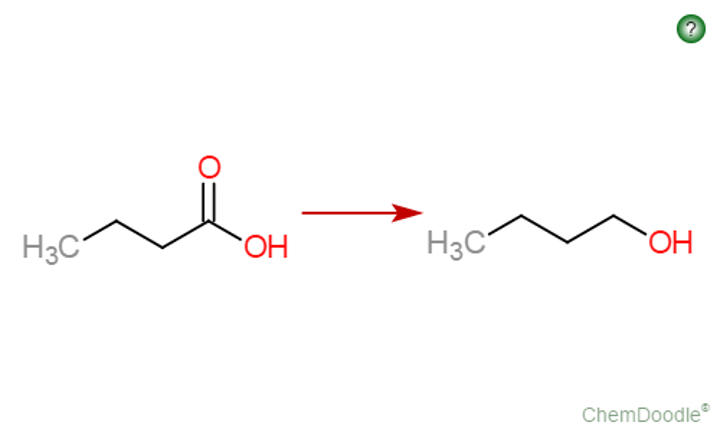
oxidation of a primary alcohol with reflux
carboxylic acid
5
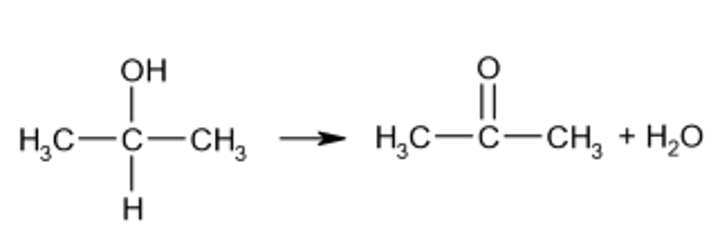
New cards
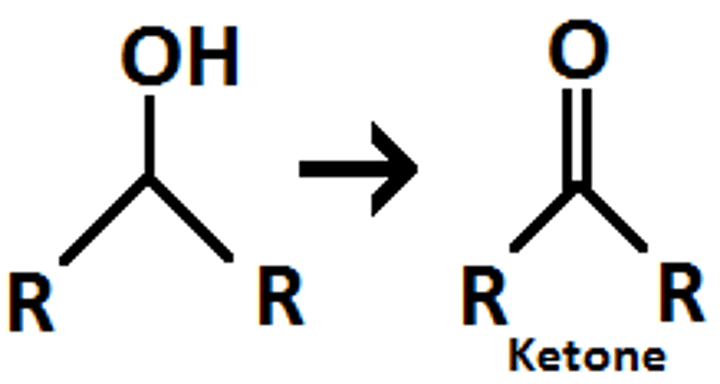
oxidation of a secondary alcohol with reflux
ketone
6
New cards
oxidation of a tertiary alcohol
no reaction
7
New cards
reduction
gaining electrons; reverse of oxidation
8
New cards
reducing agents
NaBH4 (weaker), LiAlH4 (stronger)

9
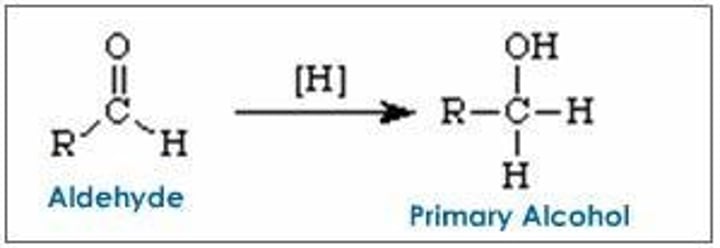
New cards
reducing agents for aldehydes
NaBH4, LiAlH4
10
New cards
reducing agents for ketones
NaBH4, LiAlH4
11
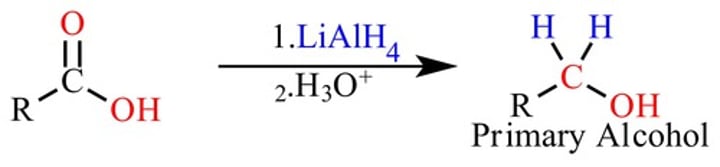
New cards
reducing agents for carboxylic acid
LiAlH4 (NaBH4 is too weak)
12
New cards
reduction of an aldehyde
primary alcohol
13
New cards
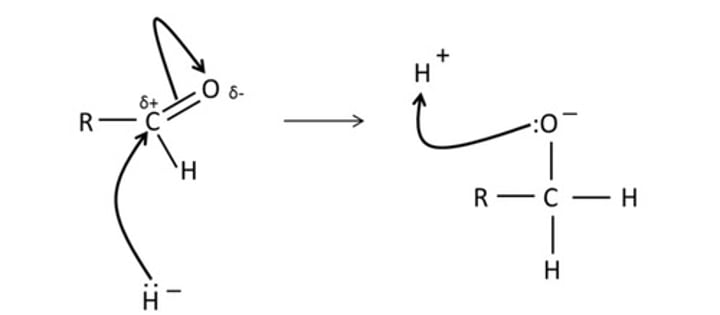
reduction of a ketone
secondary alcohol
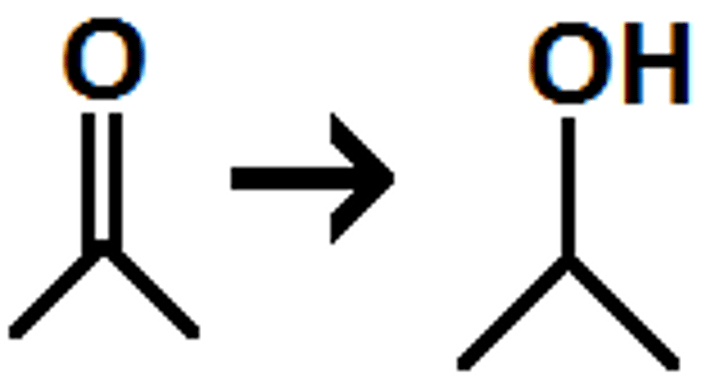
14
New cards
reduction of a carboxylic acid
primary alcohol (not an aldehyde)