Copper and its alloys
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Introduction
Cu and Cu alloys are a major group of commercial metals, ranking third behind steel and Al in production and consumption.
Non magnetic
Density: 8.96 g/cm^3
Heavier than steel
Young's modulus of 110 GPa, half of steel
Low electrical resistivity (16.78 nΩm)
High thermal conductivity (401 W/mK)
Best of any commercial metal
Very soft
FCC structure
Easy to cold work
Annealed YS: 40 MPa, cold worked YS: 200 MPa, appreciable strength by cold working
One of the very few non ferrous metals to be used unalloyed.
To improve strength, alloying elements such as Zn, Sn, Al, Si, Ni and Mn are added
With Zn becomes brass
With Sn becomes bronze
Electrical conductivity is reduced with alloying and to a lesser extent thermal conductivity.
Main impurities: O, S, Pb, P.
Cu considered noble (but its not) due to its stable temp. in the environment. Actual noble metals are gold and platinum. Cu is found as a compound combined with S
Main groups of Cu and its alloys
Elements most commonly alloyed with Cu: Al, Ni, Si, Sn, Zn
• Coppers, which contain a minimum of 99.3wt% Cu (residual deoxidisers ex P or minor alloying ex Ag, Zr, Cd, Te)
• High-copper alloys, which contain up to 5wt% alloying elements (ex Be, Cd, Cr, Fe)
• Copper-zinc alloys (brasses), which contain up to 40wt% Zn
• Copper-tin alloys (phosphor bronzes), which contain up to 10wt% Sn and 0.2wt% P
• Copper-aluminium alloys (aluminium bronzes), which contain up to 11wt% Al
• Copper-silicon alloys (silicon bronzes), which contain up to 3wt% Si
• Copper-nickel alloys, which contain up to 30wt% Ni
• Copper-zinc-nickel alloys (nickel silvers), which contain up to 27wt% Zn and 18wt% Ni
• Special alloys, which contain alloying elements to enhance a specific property or characteristic.
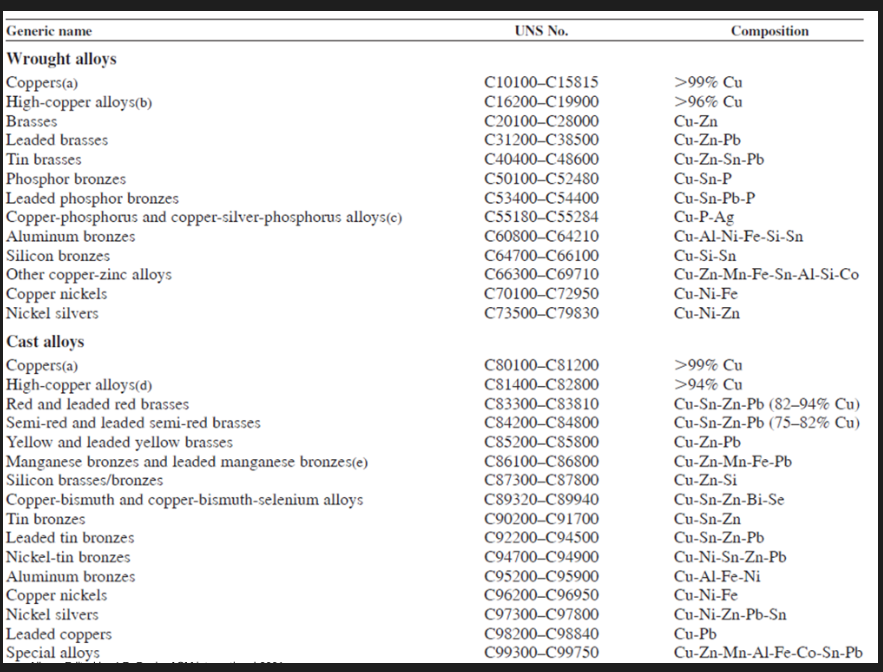
UNS numbering of Cu and its alloys
Each designation consists of 5 letters following the prefix letter C
Wrought alloys designated by numbers C1xxxx to C7xxxx
Cast alloys designated by C8xxxx to C9xxxx
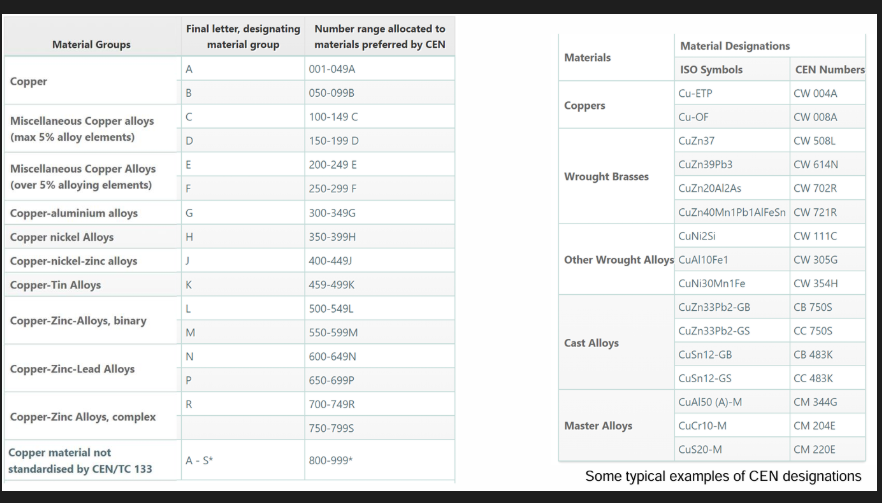
CEN European numbering of Cu and its alloys
CEN standards use a letter C followed by a second letter
W - wrought
B - ingots
C - casting
M - master alloy
Followed by 3 digits to identify materials followed by a letter that identifies classification of individual Cu material groups.
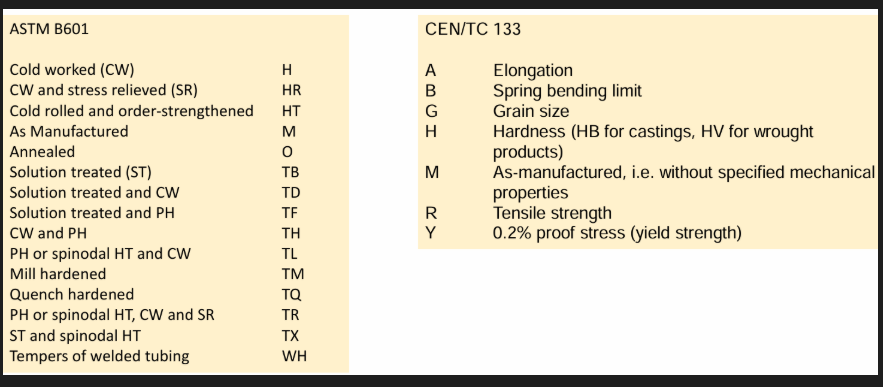
Temper designation see right
Copper alloy selection criteria
Mechanical properties
Corrosion resistance
In atmosphere, seawater, steam and in many chemical environments due to formation of protective passive film.
Can still be attacked by common reagents and environments
Some corrosive media that result in high corrosion rates of coppers include acetylene, chromic and picric acid, hydrogen peroxide (>10%), moist sulphur chloride and silver salts.
Coppers, brasses, some bronzes, and cupronickels are used for pipes, valves, and fittings in systems carrying potable water, process water, or other aqueous fluids.
Some copper alloys, however, sometimes have limited usefulness in certain environments because of hydrogen embrittlement, stress corrosion cracking (SCC) or dealloying.
Hydrogen embrittlement
Hydrogen embrittlement is observed when tough pitch coppers, which are alloys containing cuprous oxide, are exposed to a reducing atmosphere. The hydrogen will diffuse through the Cu and react with the copper oxides forming water. The water will form pressurised bubbles at grain boundaries which can lead to cracking. Most copper alloys are deoxidized and thus are not subject to hydrogen embrittlement.
Stress corrosion cracking
Stress-corrosion cracking most commonly occurs in brass (Cu-Zn alloy) containing >15% Zn. SCC requires both tensile stress and a specific chemical species (ammonia, amines) to be present at the same time. Annealing or stress relieving after forming alleviates SCC. (parts should not be bent or strained in service).
Dealloying
Dealloying affects Zn containing Cu alloys (brasses which contain >15% Zn). The more active metal is selectively removed from an alloy - a weak deposit of the more noble metal is left behind. Brasses with more than 15% Zn are susceptible to dezincification – here selective removal (leaching) of zinc leaves a relatively porous and weak layer of copper and copper oxide – unless arrested dealloying penetrates the metal. Additions of tin (0.25-2%) can improve the resistance to dezincification for brass or minor additions (0.02-0.1%) of an inhibitor element (As, Sb, or P). Dezincification of high-zinc alloys frequently results if they are used indiscriminately in fresh water service.
Electrical and thermal conductivity
Cu are good electrical and thermal conductors (more than half the use of Cu for electrical conduction, in wires and cables). All alloying elements reduce the electrical and thermal conductivity of pure Cu depending on the amount in solution and the element. Oxygen reacts with impurities creating insoluble oxides and reducing the harmful effects on electric conductivity. Electrical conductivity decreases with temp. but is independent of grain size and crystal orientation. Very small reduction (2-3%) with cold working.
Surface finish and colour
Used for aesthetic purposes due to the attractive colour combined with desirable mechanical/physical properties.
Ease of fabrication
Capable of being shaped to the required form and dimensions by any forming or forging processes. Casting alloys exist for all generic Cu families. Pure Cu is hard to cast (surface cracking, porosity). Small amounts of elements are used to improve castability (Be, Si, Ni, Sn, Zn, Cr). High workability (hot + cold working, respond to work hardening and annealing). Polished, textured, plated, coated as required. Readily assembled by joining processes. Good machiabilty. Welding, brazing and soldering readily used. Mechanical and bonding processes may be easily applied.
Strengthening mechanisms (see general notes)
Solution hardening: Zn, Ni, Mn, Al, Si listed with approximated order on increased effectiveness.
Work hardening: wrought only: most Cu alloys, depends on type and amount of alloying elements
Dispersion strengthening: used in Cu for hardening, controlling grain size, providing softening resistance. Ex Fe particles in Cu Fe alloys and Al bronzes, fine alumina particles in basic Cu matrix gives increase softening resistance
Precipitation hardening: few of most imp. Cu systems ex Be-Cu system and Cr-Cu system. produce strongest Cu alloys
Spinodal hardening: similar to PH above as it involves quenching and subsequent HT. Regular variations in composition occur in lattice with an extremely fine spacing between them. Ex Cr-Ni-Sn alloys
Pure coppers
Contain at least 99.3% Cu
Soft, ductile, very modest strength
Responds very well to work hardening
High thermal and electrical conductivity
Wrought coppers could be strengthened by CW– exposure to elevated temperatures readily anneals CW microstructure– they cannot be hardened by HT
Inherently resistant to atmospheric and aqueous corrosion and relatively insensitive to SCC.
Good fabricality (forming and joining) but casting is difficult
Trace amounts of silver or phosphorus (a deoxidizer) may be present. Silver imparts annealing resistance, while phosphorus facilitates welding.
Wrought coppers: According to oxygen and impurity contents
Oxygen free coppers
Applications requiring high electrical and/or thermal conductivity (around 100% IACS)
0.001wt%O or less
Most expensive
Produced by electrolytic refining of Cu
Used for high currents and super corrosion resistant, best of all coppers
Exceptional ductility
Low softening temp. 150
Adding:
Ag, Cd, Fe, Co, Zr imparts resistance to softening at times and temps in soldering.
Te, S for good machinability
Dispersion strengthening with Al oxides inhibits softening at elevated temps.
Applications: electric conductors/connectors, heat exchangers, microwave tubes, chemical plant equipment, gaskets
Tough pitch copper
Contain up to 0.05% O
Have Cu2O inclusions which affects strength, hardness and ductility slightly
Prone to H2 embrittlement. Heating to above 400 in an H2 containing atmosphere leaves a porous structure so cannot be used in reducing environments.
Not easily brazed
Less suitable for fabrication by CW but produces small solidification shrinkage - less scrap loss.
May contain small amount of elements to impart softening resistance.
Electrolytic tough pitch copper (Cu-ETP) has a high conductivity of pure copper (100% IACS). Contains enough oxygen to ensure that residual impurities are present as oxides rather than in solution.
Applications: electrical wires and cables, roofing and architectural trim
Phosphorus deoxidised coppers
P added to reduce oxygen in copper when in the molten state.
P reacts with dissolved O to form POs that will pass as immiscible slag
Unreacted P is dissolved in Cu
Different grades depending on amount of residual P.
Example: Phosphorus deoxidised high residual phosphorus contains 0.01% to 0.04% P. Reduction in electrical conductivity. 15% conductive than tough pitch coppers.
Can be welded without danger of hydrogen embrittlement.
Applications: piping and tubing
Types of coppers
Silver bearing copper and silver bearing tough pitch copper
Example C11300 - 100% IACS
Non heat treatable alloy
Addition of 0.05% Ag reduces conductivity by 1% but increases recrystallisation from 150 to 340 degrees.
Solder of electrical conductors to hard drawn contacts eg segments of electric motors. Solder at temps of less than 340.
Tellerium copper
Example C14500 - 93% IACS
0.5% Te improves machinability as it forms a chip breaker
Does not vastly affect conductivity
Corrosion resistant
Applications: high duty electrical contacts in machines and switch gears for ships and chemical parts, forgings and screw machines (lathe) products
High copper content alloys:
Contain up to 5% alloying elements exs: Be, Cd, Cr, Fe, Zr, Ni, Co, Sn. Used to impart higher strength, thermal stability (softening resistance) while retaining sufficient electrical conductivity.
Wrought: C16200-C19900; Cast: 81400-C82800
Retain FCC structure of Cu
Cadmium copper:
Cd is toxic and has little effect on conductivity of Cu.
Raises softening resistance, wear resistance, strength, toughness, fatigue resistance of Cu.
Resistant to arc erosion.
Non heat treatable alloy.
Strength by work hardening and solid solution strengthening.
Example: C16200 (99% Cu, 1% Cd), TS - 276-655MPa; YS 100-490MPa; 90% IACS
Applications: overhead transmission cable, overhead conductors for trams, aircraft wiring
Chromium copper:
Precipitation hardenable (cast and wrought)
Containing up to 1.2 wt% Cr for higher strength and improved thermal softening resistance 350
Electric conductivity >80%IACS.
Exs: C18200 (99Cr-1%Cr), 80%IACS (TH04 temper) and 40%IACS at TB00temper (YS 400MPa and TS 460MPa at TH04 temper)
Quenched from 1000 oC to produce a soft and ductile alloy with low electrical conductivity.
Dissolved Cr will hinder flow of electrons, same for all alloys.
Heating for 2 h at 500 oC restores electrical and mechanical properties.
Excellent workability coupled with medium to high conductivity.
Applications: resistance welding electrodes; current carrying shafts; moulds; circuit breaker parts; trolley wire for high speed trains.
Beryllium copper:
Cast and wrought alloys
Precipitation hardenable containing up to 2%Be (wrought); 2.85% (cast), and sometimes small amounts of Co, Ni and Fe.
Can be formed in soft condition.
High strength copper alloys.
Two classes:
High strength alloy:
Ex: C17000 (98Cu-1.7Be-0.3Co) YS THO4 1200MPa 25%IACS
Used when mechanical properties are needed rather than electrical properties. Softened by heating at 800 oC and quenched to allow cold work and machining. Heat treated at 300-320 oC for 2 h.
Applications: pressure gage, bourdon tubes, hand tools in explosive environments (no sparks!), flexible bellows, fasteners,), welding equipment, plastic injection moulds (cast); pitot tube housing in high speed aircraft, golf club heads.
High conductivity alloy:
Ex: C17600 (Cu 0.25-0.5Be 1.4-1.7Co 0.9-1.1Ag) YS TH04 690-825MPa; 50-60%IACS.
Applications: A high-conductivity alloy designed especially for resistance welding electrodes for spot, seam, flash, and projection welding methods; electrical connectors, clips.
Brasses (general)
Alloy of copper and zinc (up to 50%) in various amounts to get a range of brasses with varying properties
>15% Zn, alloy prone to SCC dealloying
Both wrought and cast alloys
Pb provides high machinability and Sn is added to high Zn brasses to improve corrosion resistance.
Subgroup of high strength brasses alloyed with Mn, Fe, Sn, Al, Si and/or Co
Brasses most common cast copper alloys due to their excellent castability and good combination of strength and corrosion resistance
Pb provides pressure tightness by sealing shrinkage pores
Applications: decoration, low friction (locks, gears, bearings), good acoustic properties (bells, instruments)
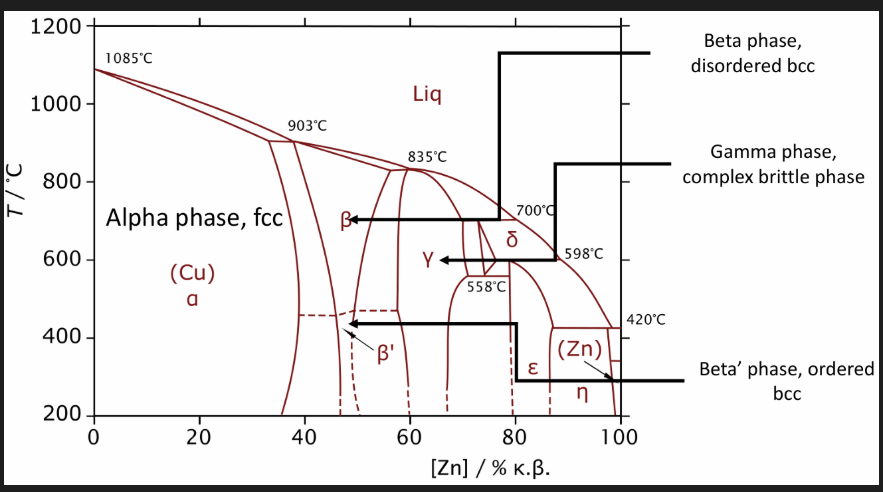
Wrought brasses
Common brasses:
Zinc is the primary alloy
Low zinc retains FCC (alpha)
High Zn (>39wt% Zn) like muntz comprises of the hard BCC beta’ phase
Between 32%-39% Zn may have a duplex alpha + beta’ structure, easy to hot work and machine
Increasing Zn produces stronger alloys but with a decrease in corrosion resistance
Produced as sheets for stampings (springs, switch components), tubes (drains, plumbing), and rod (fasteners and forgings)
Adequate corrosion resistance in most atmospheric environments.
Subject to dezincification in stagnant and acidic aqueous environments.
May fail by SCC in presence of moist ammonia, amines, mercury components.
High electric conductivity example: Cartridge brass (30% zinc, used in common electrical hardware has conductivity of 28% IACS, bullet cases strong and ductile)
Selection based on formability (cartridge optimum), corrosion resistance (low Zn%), colour (reddish pink to pale yellow with increasing Zn content)
Increase zinc, become cheaper
Examples:
Gilding Metal (<5% Zn – C21000) – ( Excellent CW; Good HW) coins, medals, fuse caps, plaques, jewelry base
Commercial Bronze (~10% Zn – C22000) – ( Excellent CW, Good HW) screen cloth, screws, rivets, marine hardware
Jewelry Bronze (~12.5% Zn – C22600) - ( Excellent CW, Good HW) fasteners, costume jewelry, base for gold plate
Red Brass (~15% Zn – C23000) - ( Excellent CW, Good HW) fasteners, conduit, heat exchanger tubing, plumbing pipe, radiator cores
Low Brass (~20% Zn – C24000) ( Excellent CW) – battery caps, musical instruments, bellows, clock dials
Cartridge Brass (~30% Zn – C26000) – ( Excellent CW) radiator tanks/cores, lamp fixtures, fasteners, locks, hinges, ammunition components, pluming accessories, rivets
Yellow / standard Brass (~ 35% Zn – C27000) – ( Excellent CW) uses same as cartridge brass but not for ammunition.
Basis Brass (~37% Zn) Cheapest of the cold working brasses but hot works well. It lacks ductility and only capable to withstand simple forming operations
Muntz Metal (40% Zn – C28000) – ( Excellent HW) Not suitable for cold working. Relatively cheap. Architectural panels sheet, large nuts and bolts, heat exchanger tubing, brazing rod, hot forgings
Wrought leaded (Cu-Zn-Pb):
Lead (0.5 - 3%) added to provide high machinability by acting as a chip breaker and tool lubricant.
Available as rod, bar, shapes and tubing.
Ar added to inhibit dezincification (C35330)
Free-cutting brass (C36000, 61.5Cu-3Pb- 35.5Zn) containing 3% Pb is normally the first choice for a copper-base screw-machine material (100% machinability). (Much better machinability than leaded steel, with similar properties and superior corrosion resistance – brass screw machining products cost can be lower than for leaded steel (easier machining, no need for electroplating)
For products that require both machining and cold forming, reduced-lead (2% Pb) copper alloys such C35300 (62Cu-1.8Pb-36.2Zn) should be considered.
Forging Brass C37700 (59Cu-2Pb-39Zn) have excellent HW and is primarily specified for corrosion- resistant forgings, such as valves and fittings, architectural hardware, and specialty fasteners. A modest lead content makes the alloy free-cutting. Like most brasses, it can be finished to a high luster and readily accepts decorative electroplated coatings.
Wrought tin brasses (Cu-Zn-Sn)
These are high-zinc brasses containing tin for better corrosion resistance and somewhat higher strength than Cu-Zn brasses.
Tin, like arsenic, antimony, and phosphorus, reduces susceptibility to dezincification.
Tin brasses have good hot forgeability and reasonably good cold formability.
In rod form, they can be cold headed to produce high strength fasteners and similar parts.
Alloy C42500 (88.5Cu, 2.0Sn, 9.5Zn) is supplied as strip for fabricating into electrical connectors, springs, and related products.
Leaded Tin Brasses alloys (ex leaded Naval brass C48500, 60Cu 1.75Pb 37.5Zn 0.75Sn ) are free machining.
The admiralty brasses (ex C44300 71Cu 28Zn 1.0Sn)) and naval brasses (ex C46400, 60Cu 39.25Zn 0.75Sn) are used for corrosion-resistant mechanical products.
Leaded naval brasses (up to 1.75Pb) are supplied in rod form for marine hardware, pump shafts, valve stems, and corrosion resistant screw-machine parts
Cast brasses
Red and Leaded Red Brasses – Cu-Zn-Sn-(Pb) – for alloys with <8%Zn appearance has a red copper like colour; moderate strength; fcc; electric conductivity adequate for electromechanical equipment (pole-line hardware); leaded red brasses contain up to 7%Pb provides pressure tightness and also improves machinability but high levels reduces properties at high temperature. High aqueous and atmospheric corrosion resistance (plumbing, valves, pump housings, impellers, statuary). Workhorse alloy is C83600 known as 85-5-5-5 (85Cu-5Sn 5Pb-5Zn. TS255MPa; YS 117MPa, El 30%)
Semi red and leaded semi red brasses – Higher zinc content (up to 15%) – lower corrosion resistance and cost; lighter colour; little effect on strength; mainly α phase (fcc); Used for plumbing fixtures, fittings, faucets and low pressure valves. Ex (C84400, 81Cu-9Zn- 3Sn-7Pb TS235MPa, YS105MPa, El 26%)
The yellow and leaded yellow brasses – 20-40%Zn; microstructure range from α (fcc) to substantial amounts of β. β is a potent strengthener which impairs slightly ductility at RT but markedly improves ductility near solidus (suitable for permanent moulding). Pleasing light colour/polishable. Lower corrosion resistance and cost than semi red brass. Uses: decorative hardware, architecture trim, plumbing fixtures. Ex (C85800, 63Cu-35Zn-1Sn-1Pb, TS380MPa, YS205MPa, El 15%)
High-Strength Brasses (Manganese bronzes) – Cu-Zn-Fe-Al-Mn; strongest as-cast copper base materials; strength from high β phase content; Al stabilises β at lower zinc content (ex C86200, 64Cu-24Zn -5Al-4Mn-3Fe, TS 655MPa, YS 330MPa, El 20%) that in binary phase diagram (39.5%); Fe refines grains and add to strength; Mn improves castability but also strength. Used for gears, bolts, valve stems and other mechanical products high-strength, good wear resistance, and reasonably good corrosion resistance. When feasible these have been relaced by equally strong but more corrosion resistant Aluminium bronzes.
Silicon brasses/Bronzes – Cu-Zn-Si (Si <5%) favourable low melting point and high fluidity alloys (suit most casting methods); moderate to high strength and very good aqueous and atmospheric corrosion resistance, although susceptible to stress corrosion cracking (SCC) in severe environments. Considered for lead free replacement of common plumbing brasses but restricted by their limited machinability. Uses: pole-line hardware; intricately shaped pump and valve components, bearings, bells ex C87610, 90Cu-4Zn-4Si, TS 380MPa, YS 170MPa, El 30%)
Copper-Bismuth and Cu-Se-Bi Brasses – low lead alloys used in food processing and potable water applications ex faucets; plumbing fixtures – developed to minimise Pb leaching in potable water while replicating high machinability and pressure tightness of leaded brass.
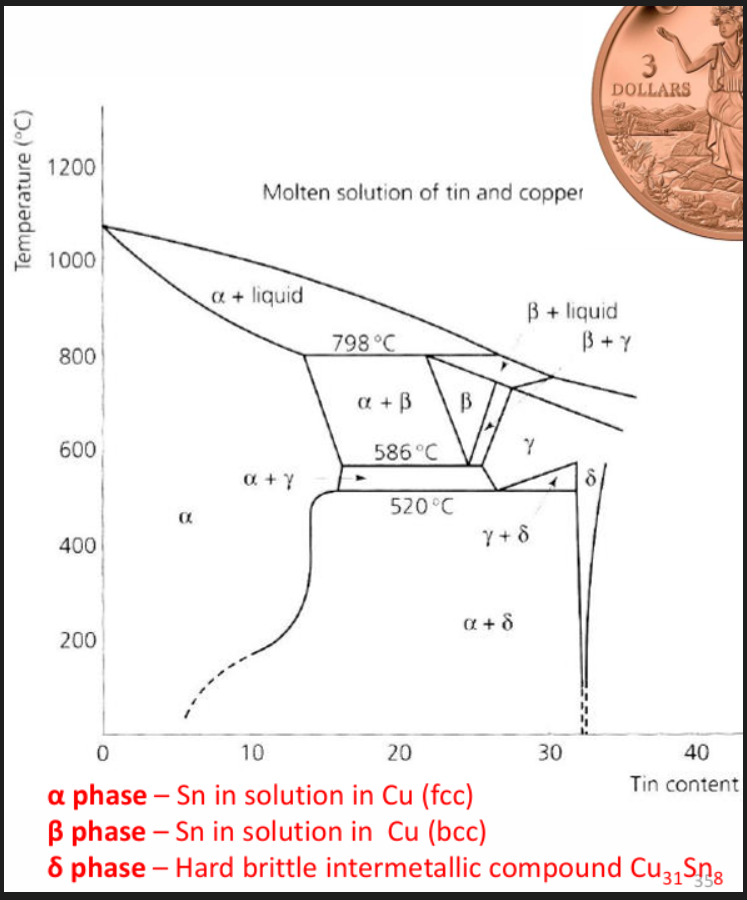
Bronzes
Bronzes are copper alloys in which the major alloy addition is neither Zinc nor Nickel. Generally Bronzes are classified by their major alloying elements. There exists both wrought and cast Bronzes. Used in marine, automotive, aircrafts etc
For wrought alloys with up to about 10%Sn, a solid solution of Sn and Cu exists. This has an fcc structure and is called the alpha phase. Sn is a strong solid solution strengthener. Tin is very soluble in copper. Alpha bronze tends to be very ductile and can be easily cold worked.
In the as-cast structure δ phase could be present even at Sn of 8%. Hard δ is important for wear resistance (bearing applications). Zn is sometime added to improve the soundness of castings for high pressure water valves.
1% of tin gives a higher strengthening effect than 1% Zn. Tin bronzes are generally stronger than brasses.
freezing range gets wider when tin content increases, casting more difficult, no casting defects.
Wrought copper bronze alloys
• Tin/Phosphour Bronzes (Cu-Sn-P)
Alloy of Copper and tin with a deoxidiser (P) Also referred to as phosphor bronzes. Typically 0.5-11%Sn and 0.1 to 0.35P
Characteristics: Superb spring qualities; high fatigue resistance, excellent formability; excellent solderability; high corrosion resistance.
Applications: Primarily as strip for electrical products; corrosion-resistant bellows; diaphrams; spring washers.
Ex Cu-1.25Sn, YS 100 to 350MPa via CW, 48.4%; Cu-5Sn, YS 130 to 550MPa, El 60-2%; Cu-10Sn, YS annealed 190 to CW 550MPa, Duc 70 -4%).
• Leaded Tin/Phosphour bronzes (Cu Sn-P-Pb)
(leaded Tin or phosphour bronze)
Characteristics: High strength together with high fatigue resistance and good machinability and excellent corrosion resistance (especially in seawater)
Applications: Thrust washers; cam followers; sleeve (journal) bearings, bushing Ex Cu-4Sn-4Pb-4Zn, YS 130 – 430MPa, El 50 -16%. Lead: machinability, prevents galling when lubrication breaks.
• Aluminium Bronzes (Cu-Al)
• Silicon bronzes (Cu-Si)
Bronzes with Silicon as their main alloying element (1-4%)
Resemble lower-Aluminium bronze in mechanical properties- TS up to about 700MPa
Good corrosion resistance but lower SCC resistance than aluminium bronzes.
Excellent weldability (used as filler wires)
May include other elements: Fe, Zn, Mn, Ni, Pb.
C65100, 98.5Cu-1.5Si Annealed to WH: TS 275-620; YS 106-460MPa
C65500, 97Cu-3Si Annealed to WH: TS 400-745MPa; YS 300-425MPa
Applications: Aircraft: hydraulic pressure lines in aircraft,
- Hardware: bolts, hinges, nails, screws, pole-line hardware, contact springs, connectors, welding rod
- Marine: propeller shafts, marine hardware
- Industrial: heat exchanger tubes, chemical equipment, cable
• Copper-Nickel alloys (Cu-Ni)
Ni addition improves strength, oxidation, and corrosion resistance. It greatly increases electrical resistivity of Cu. used extensively in marine environments: cooling systems in ships
The α phase is present over the entire range all Cu-Ni alloys have good ductility.
Cu-Ni alloys are amongst the most corrosion resistant and thermally stable of all Copper alloys. Virtually immune to SCC.
They exhibit high oxidation resistance in steam and moist air.
Moderate to high strength is retained well at high temperatures.
Good hot and cold formability
Biofouling resistance (highest for the low Ni alloys)
C70600, 88.6Cu-10Ni-1.4Fe, TS 300– 410MPa YS 110-400MPa El% 40– 3 via CW, 9%IACS
C71500 70Cu-30Ni, TS 380-520MPa YS 140– 480MPa El% 45– 15viaCW
C71700 67.8Cu-0.7 Fe-31 Ni-0.5 Be, TS 480-1380MPa YS 207-1240MPa, El% 40-4. Be makes alloy heat treatable, PH,
Applications: Marine (resists seawater, SCC and biofouling) such as piping, heat exchangers and condensers. High strength constructional parts for seawater corrosion resistance, mooring cable wire, bolts (C71700)
• Nickel silvers (Cu-Ni-Zn)
Cu-Ni-Zn alloys that generally contain more Zn than Ni. (thought as Ni brasses)
Named for their bright silvery luster (decorative hardware, electroplated tableware, optical equipment musical instruments)
Moderate to high strength and good corrosion resistance. (food and beverage handling equipment)
Similar characteristics to α-brasses but Ni gives them superior tarnish and SCC resistance. Excellent cold workability
C74500, 65Cu-25Zn-10Ni YS150-750MPa El%50-1
C75200, 65Cu-17Zn-18Ni, YS 170 to 620MPa El% 45-3
Other uses: rivets, screws, resistance wire, costume jewellery, zippers
Cast copper bronze alloys
• Tin Bronzes (Cu-Sn-P)
Tin increases strength via solid solution and increases corrosion resistance (Many Bronze age artefacts are still intact after 3500years!) very resistant to corrosion
At circa 520°C, the alpha Cu-Si solid solution could take up to ~16%Sn. Low temperature transformations are usually very sluggish.
Increasing Sn broadens the freezing range – hence increase care required in the design of castings.
Tin bronzes are stronger and more ductile than red and semi red brasses and can be used at higher temperature than leaded alloys. They have high wear resistance and low friction against steel.
Applications: bearings, gears, piston rings, valves, fittings, bells, steam fittings, pump impellors
Ex: C90500, 88Cu-10Sn-2Zn, TS 317MPa, YS 152MPa, El 30%
• Leaded Tin bronzes
Principal functions of lead in Cu-Sn and Cu-Sn-Zn is to improve pressure tightness and machinability.
High Tin bronzes often require some lead (typically 1% is sufficient) to seal interdendritic porosity. More is sometimes added to improve machinability and bearing properties. (Lead reduced tensile properties hence a balance of properties is required).Lead reduces tensile properties
Ex C92200 (88Cu-6Sn-1.5Pb-4.5Zn) TS280MPa YS 110MPa El 45%(steam bronze) – corrosion resistant valves, pressure retaining parts at temperatures up to 290°C.
Can be soldered and brazed but not welded.
High-leaded tin bronzes (ex C93800 , 79Cu-6Sn-15Pb, TS 221MPa YS110MPa El% 20) are used for sleeve bearings.
• Nickel-Tin Bronzes
These cast bronzes combine strength, toughness and high corrosion resistance. can be cooled rapidly, aged respond to strengthening mechanism called spinodal decomposition
Can be cast via most foundry processes.
Soft and ductile in the as-cast or solution annealed and quenched condition.
Low temperature aging (315°C) cause spinodal decomposition sharply increasing mechanical properties.
Exs
C94700, 88Cu-5Ni-5Sn-2Zn, TS 345 (590MPa) YS 169 (410MPa) El% 35 (10) (Heat treated)
C94800, 87Cu-5Sn-5Ni, TS 310 (410MPa) YS 160 (210MPa) El% 35 (8) (Heat treated)
Applications: Specialty bearings, pistons, nozzles, mechanical actuators, shift forks
• Aluminium Bronzes
• Copper-Nickel alloys (Cu-Ni)
• Exceptional corrosion resistance in seawater (highest for 30%Ni)
• Virtual immunity to SCC (even in ammonia, amines, nitrides)
• Fe, Cr, Nb and/or Mn added for improve strength and corrosion resistance.
• High strength and toughness from low to elevated temperatures
• High biofouling resistance (highest for low nickel alloys)
Exs
C96200 85Cu-10Ni-1.4Fe-1.5Mn-0.5Si-0.8Nb, TS 310MPa, YS 170MPa, El% 20., 11% IACS
C96400 69Cu-30Ni-0.9Fe-1.5Mn-0.5Si-1Nb, TS 470MPa YS255MPa El% 28, 5%IACS
C96600 67.5Cu-30Ni-0.5Be-1Fe-1Mn TS 515 to 825MPa, YS 260 to 515MPa via HT (TF00 temper)
Applications: Aboard ships, offshore platforms and coastal power plants (valves, pump bodies, flanges, elbows. Best materials for evaporative desalination plants; considered for spent nuclear fuel burial containers.
• Nickel silvers (Cu-Ni-Zn)
Cu-Ni-Zn-Pb-Sn alloys (some are actually leaded Ni brasses)
Low to moderate strength.
Good castability. Sn, Ni gives good aqueous corrosion resistance; Pb provides pressure tightness and machinability
Uses: Ornamental alloys, architectural trim, musical instruments, marine hardware, low pressure valves and fitting for the food, diary and beverage industries.
C97300, 56Cu-12Ni-20Zn-2Sn-10Pb TS240MPa YS120MPa El% 20
C97600, 64Cu-20Ni-8Zn-4Sn-4Pb TS 310MPa YS165MPa El% 20
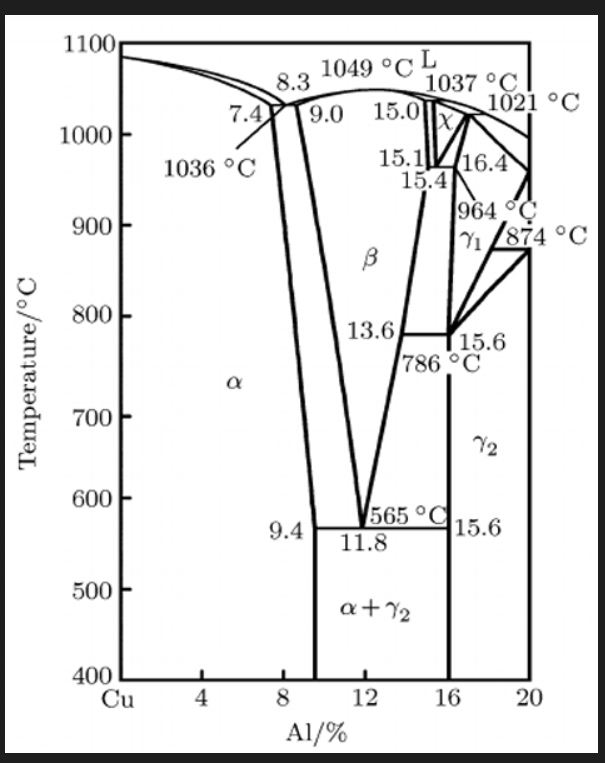
Cu-Al (Aluminium bronzes, wrought and cast)
Bronze in which Al (often 5-11%) is the main alloying element. Alloying elements such as Fe, Ni, Mn and Si are sometimes added.
Good combination of high strength and excellent corrosion resistance (seawater, chlorides, dilutes acids ex H2SO4, HNO3, HCl, HF).
Less susceptible to crevice corrosion than stainless steels and resist both pitting and SCC. The high alumina content in the protective oxide film contributes to their high erosion-corrosion and cavitation resistance.
Readily weldable; good wear resistance; Resist biofouling; non sparking.
Bronzes with <9.5%Al are strengthened via solid solution strengthening, cold working (wrought only) and via precipitation of iron- or nickel-rich kappa phases .
Wrought alloys containing <9.5% Al can reach TS 480-690MPa depending on composition and temper.
Wrought Alloys with 9 to 11%Al can be quenched and tempered much like steel to produce TS >1000MPa.
Ex C63000 (82Cu-10Al-5Ni-3Fe)
Cast alloys
Ex C95500, 81Cu-11Al-4Fe-4Ni-3.5Mn can reach TS 760MPa and YS 415MPa when HT
High strength to weight ratio (Aluminium reduced density of alloy) – landing gear bearings sometimes made from nickel-aluminium bronze.
Applications: Marine hardware; shafts, pump and valve components for handling seawater and other corrosive fluids, heavy duty sleeve bearings, seawater pipes, propellors and propellor hubs, non-sparking tools.
Looking at phase diagram:
• The Cu-Al shows that Al forms a solid solution with Cu (α phase - fcc) when added to up to 9.4% at 565ºC. Al is a potent solid solution strengthener. responsive to strengthen by CW
• Above 8%Al alloys can form the β phase (bcc) at high temperature which upon slow cooling undergoes a eutectoid reaction to α and ϒ2. gamma 2 phase: brittle, intermetallic, not desirable in seawater applications, selectively corroded in such environments
• Cooling slowly through eutectoid produces and alternate layers of α and ϒ2 (like pearlite).
• Rapid cooling will result in a martensitic transformation of the β phase to β’ phase (hexagonal crystal structure) with a needle like structure.
• Unlike in steels, the martensite is slightly less hard than the pearlite. (80 vs 70 HRB). Also tempering of the martensite below 400 °C will increase hardness to around 90HRB (fine dispersion of ϒ2 ).
• Alloys containing γ2 phase are unsuitable for cold working but excellent for casting and can be hot worked.
Quench: martensite and beta prime, temper we get alpha + dispersion of gamma 2
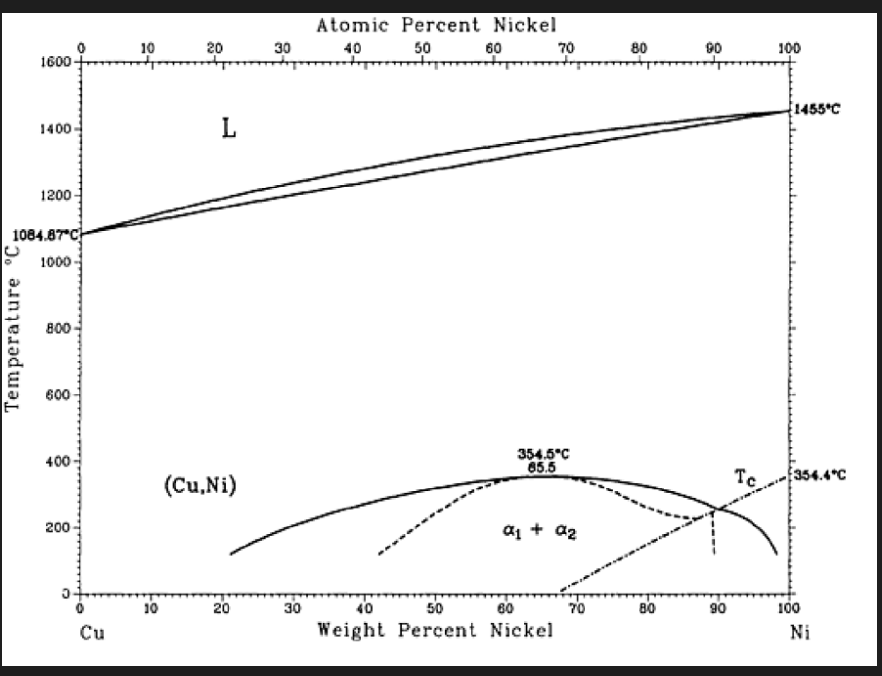
Cu-Ni phase diagram
• Complete solubility in both liquid and solid phases.
• The behaviour below the dotted line on the both right corner is ferromagnetic and above it is paramagnetic.