Reactions of Phenols
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is a phenol?
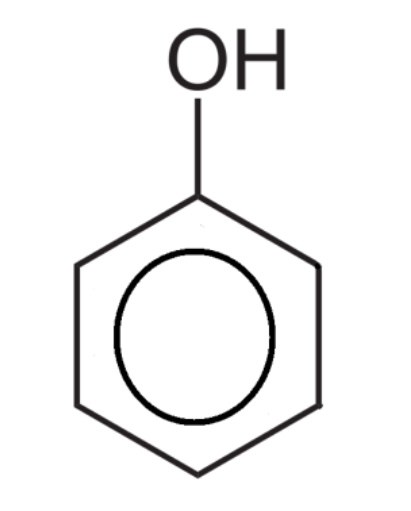
Phenol is an aromatic compound with an –OH group bonded directly to a benzene ring.
Briefly describe the acidity of phenols.
Phenols are weakly acidic.
Which is least and most acidic: phenols, carboxylic acids, and aliphatic alcohols?
Aliphatic alcohols, Phenols, Carboxylic acids.
Least acidic
Most acidic
Explain why phenol is slightly acidic.
The oxygen atom in the hydroxyl group has a lone pair of electrons that interact with the pi-bonding system in the benzene ring.
The lone pair of electrons on the oxygen atom are drawn into the pi cloud of the benzene ring.
This causes the C-O bond to becomes shorter and stronger.
The O-H bond weakens and breaks; dissociation occurs and an hydrogen ion can be donated.
What is the name of the ion formed when a hydrogen ion is donated by a phenol?
Phenoxide ion.
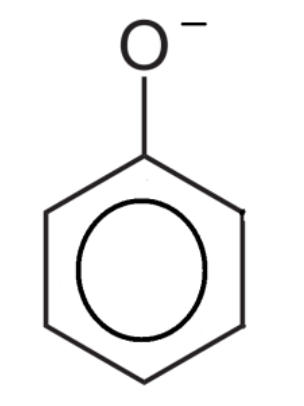
Can phenols react with sodium hydroxide?
Yes.
Can phenols react with sodium carbonate?
No- it is not acidic enough.
Can carboxylic acids react with sodium carbonate?
Yes.
Carboxylic acids react with both NaOH and Na2CO3 and therefore sodium carbonate can be used to distinguish between phenols and carboxylic acids.
How do phenols react?
By electrophilic substitution due to the delocalised electron system.
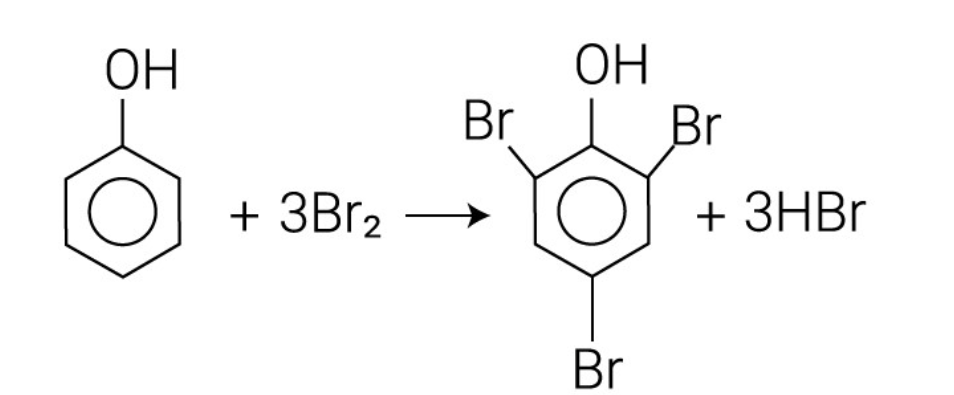
Describe the reaction of phenols with bromine water.
Phenol reacts with bromine water by the substitution of 3 hydrogen atoms to form 2,4,6-tribromophenol.
2,4,6-tribromophenol forms as a white precipitate and the orange bromine water is decolourised.
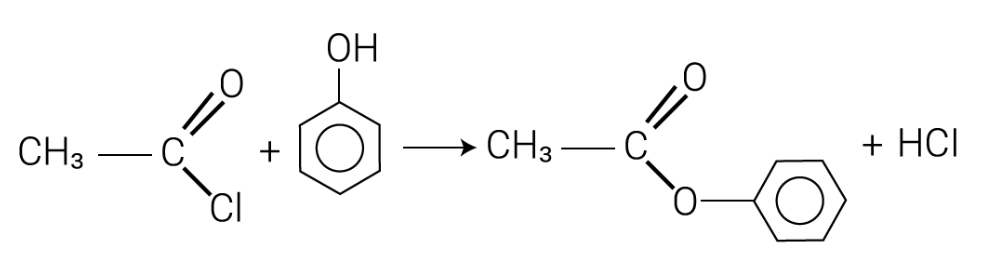
Describe the reaction of phenols with acid halides such as ethanoyl chloride.
Phenols react with ethanoyl chloride to form aromatic esters, such as phenyl ethanoate, in addition-elimination reactions.
State the 2 ways to test for phenols.
Reaction with bromine water: phenols produce a white precipitate with bromine water. The bromine water is decolourised.
Reaction with iron (III) chloride: The addition of yellow iron(III) chloride solution to phenol will give a purple colour.