Unit 2: Chemical Formulas, Equations, and Reactions
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Coefficient
A number in front of a chemical formula in an equation that indicates how many molecules of each reactant and product are involved in a reaction.
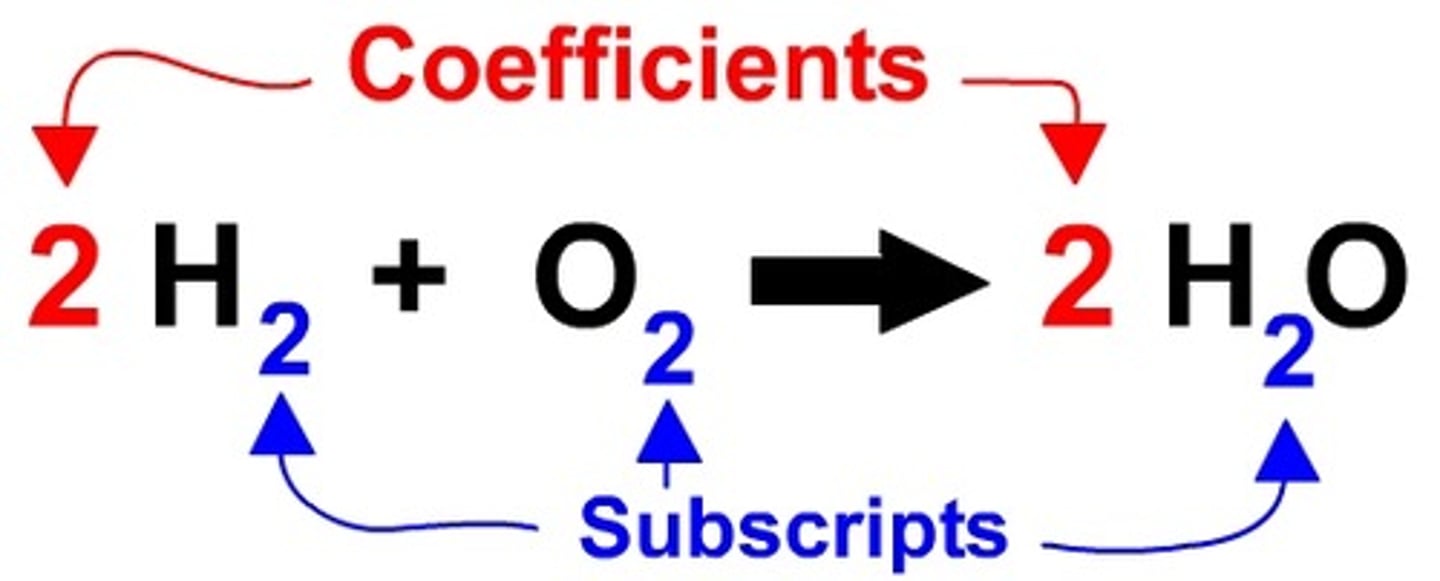
Subscript
A number in a chemical formula that tells the number of atoms in a molecule or the ratio of elements in a compound
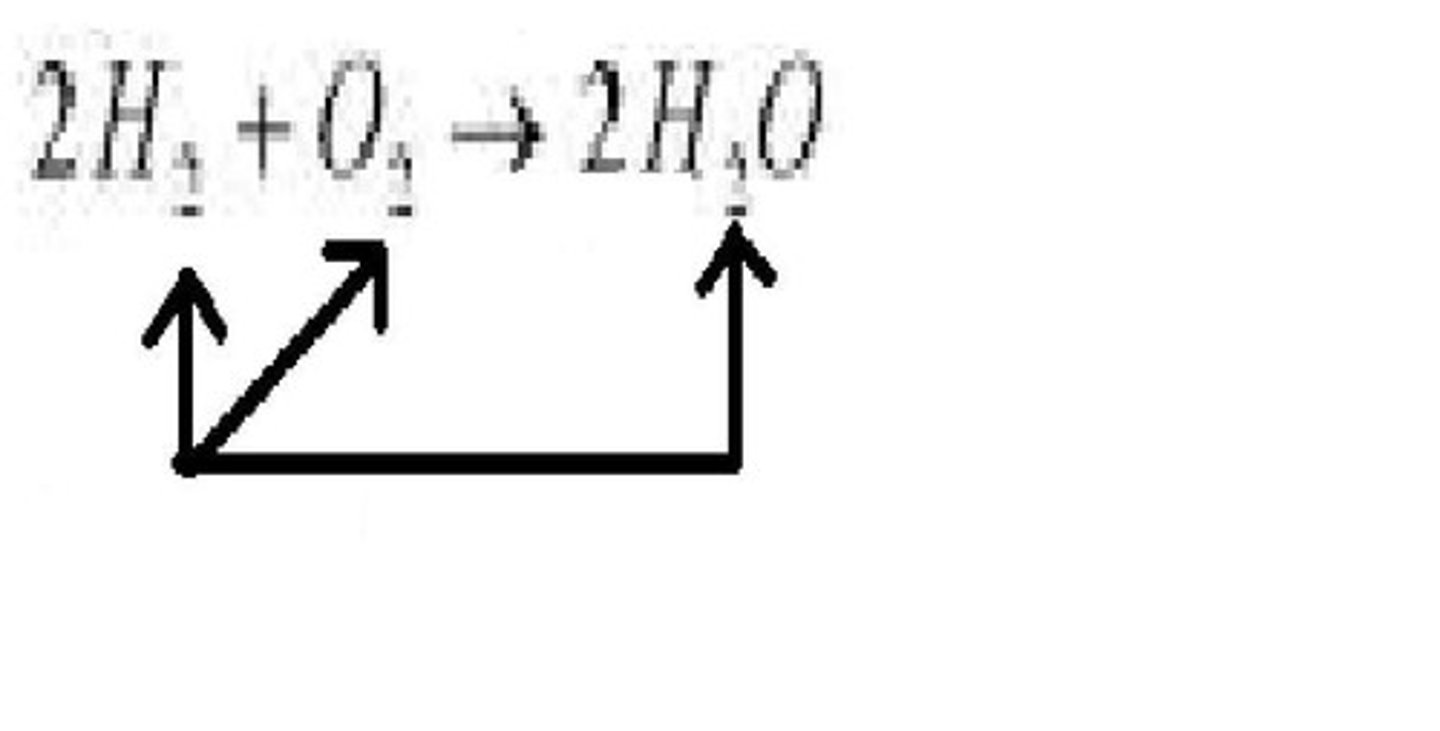
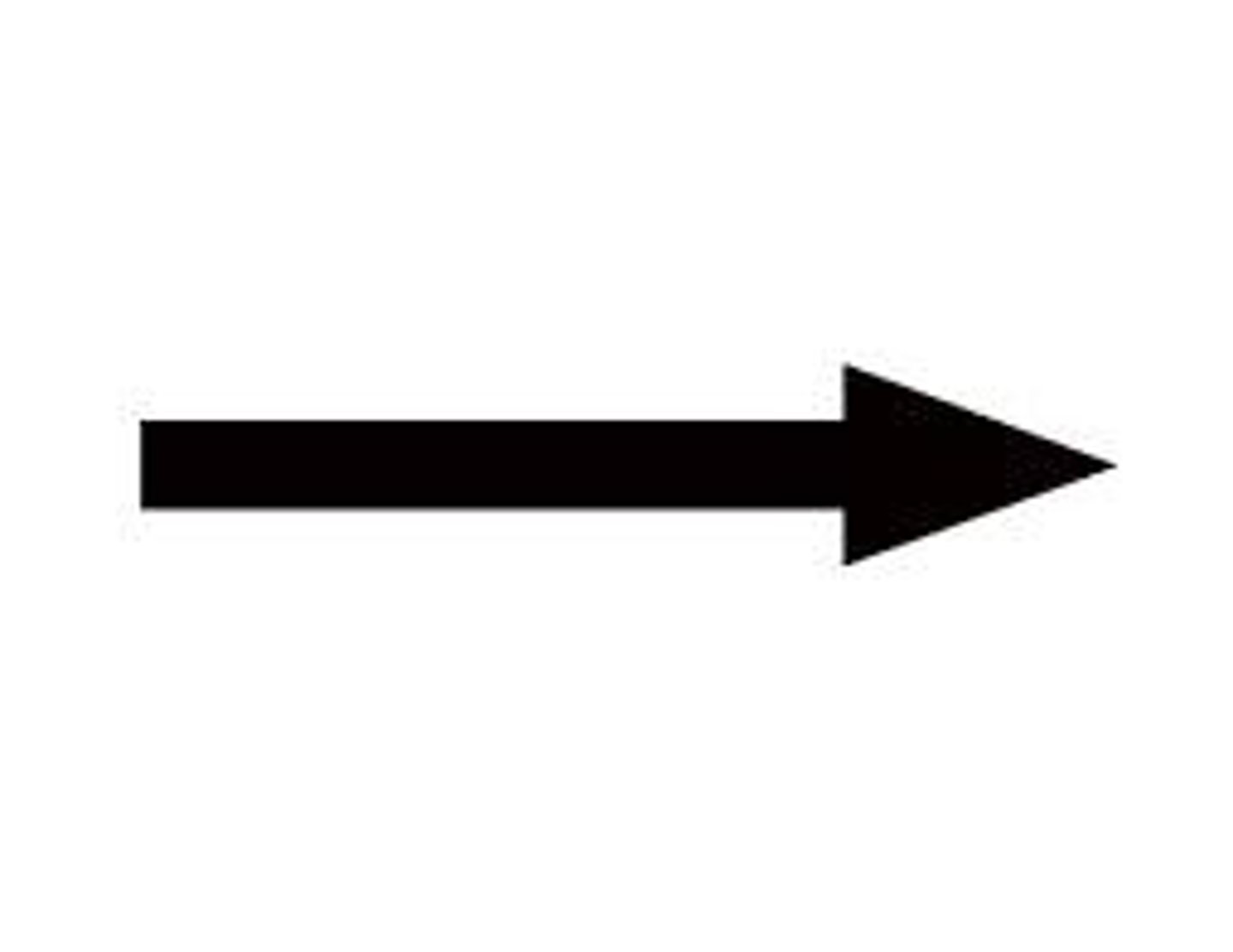
arrow
yields or produces; indicates a chemical reaction
chemical formula
A combination of chemical symbols and numbers to represent a substance
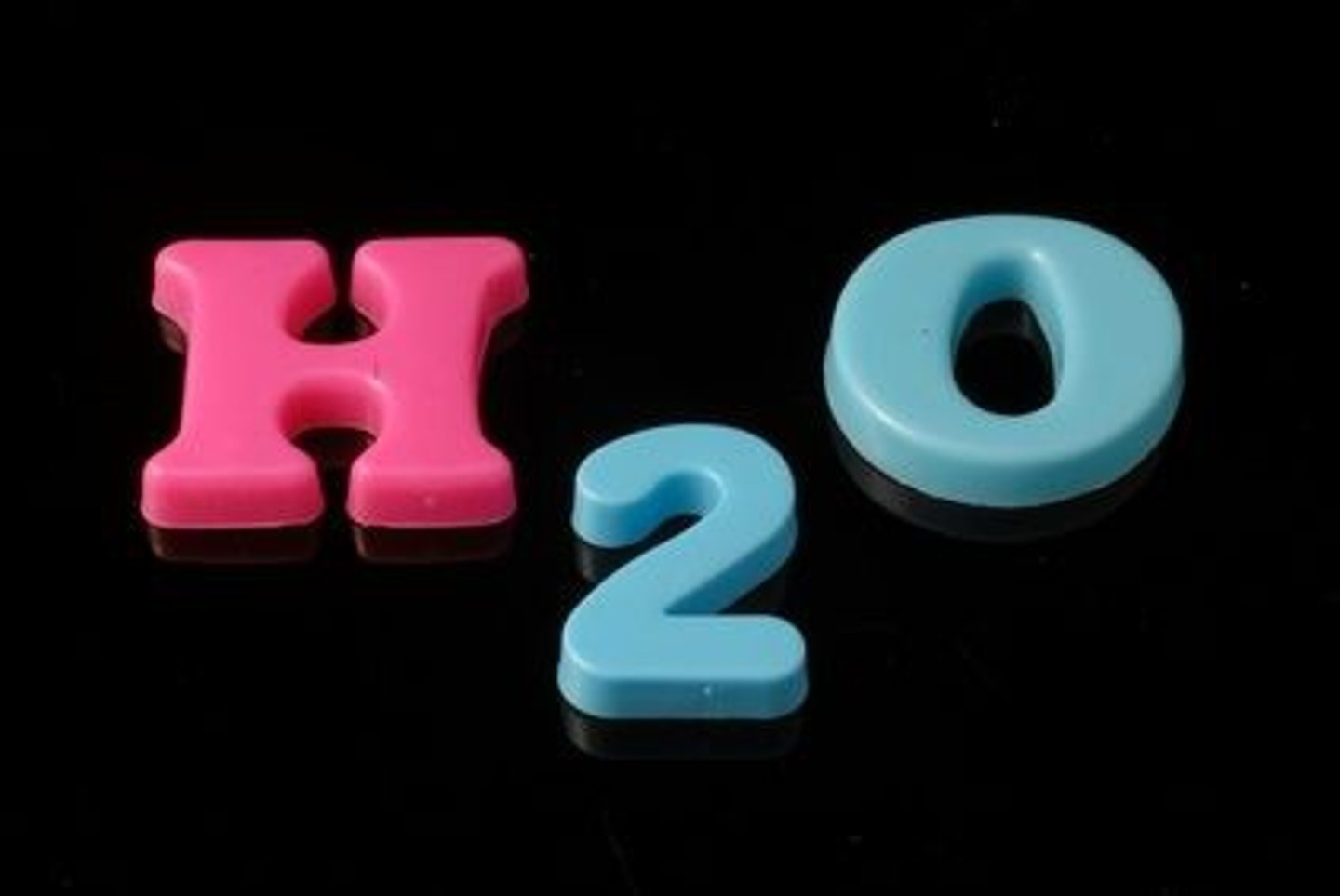
Chemical equation
a way to describe a chemical reaction using chemical formulas and other symbols
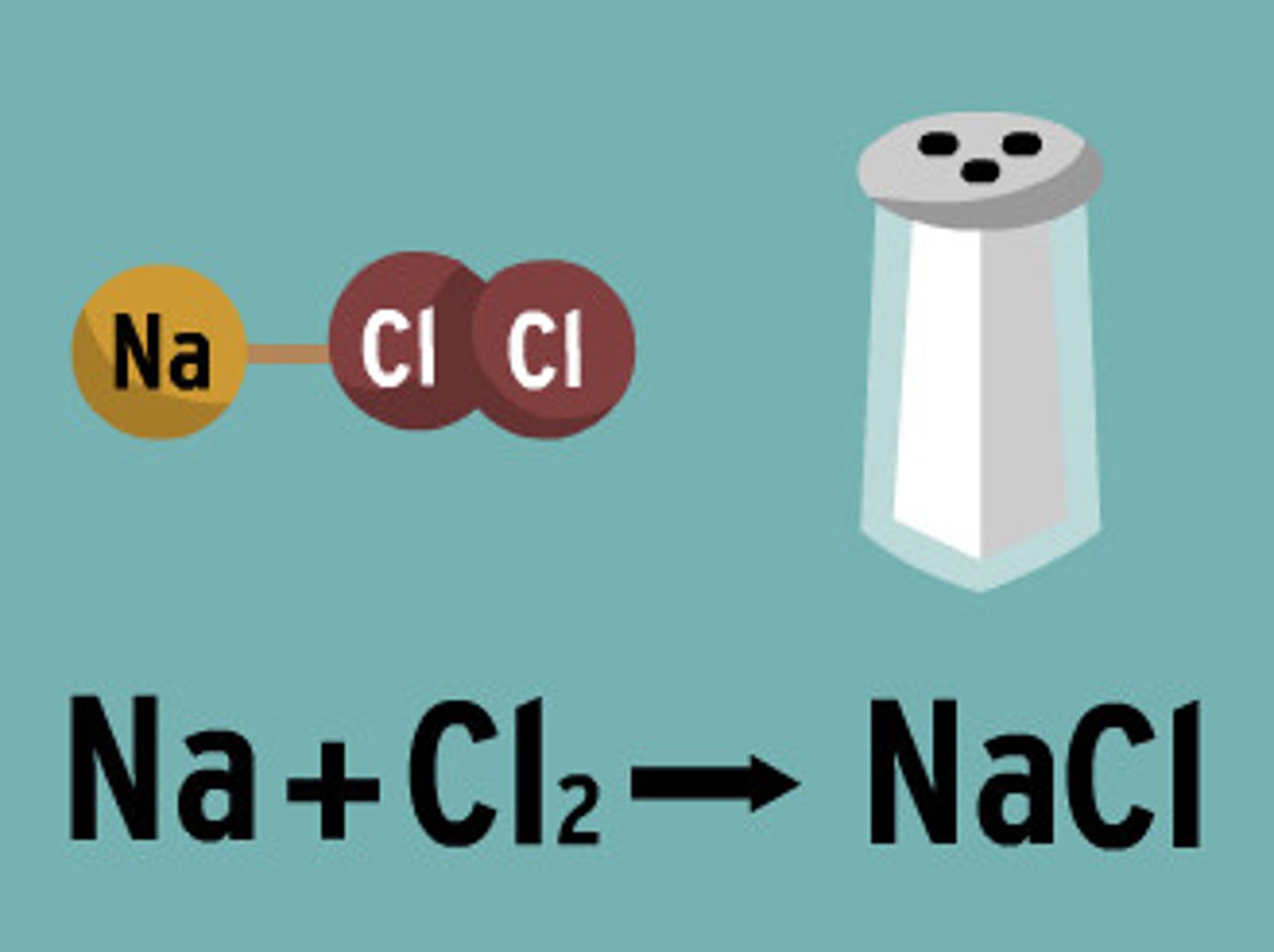
Reactants
A starting material in a chemical reaction
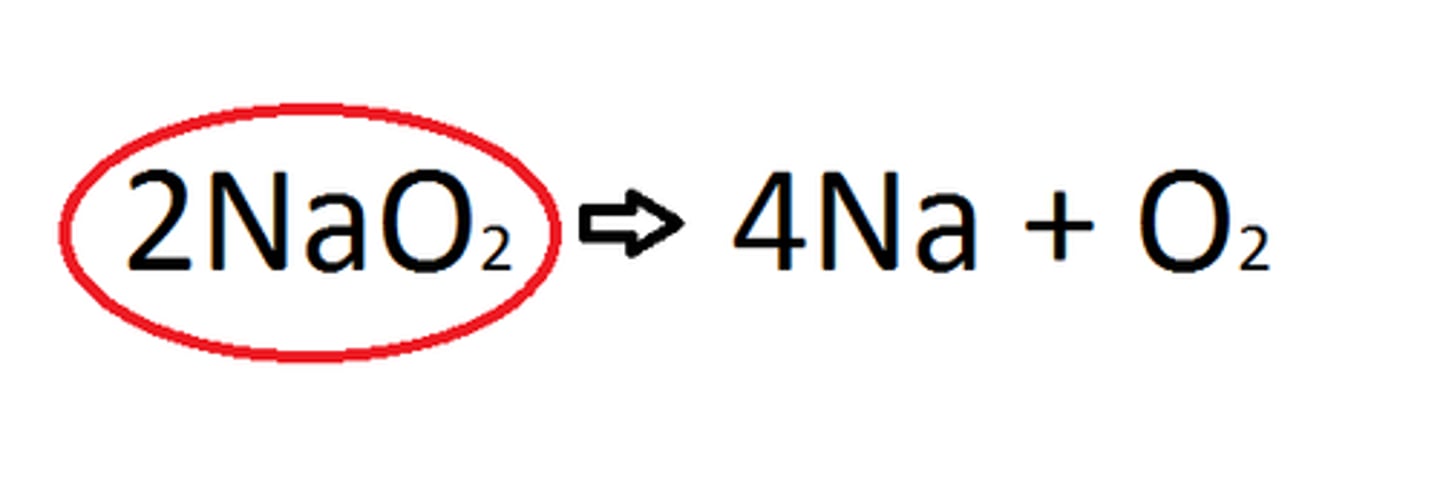
Products
Ending materials in a chemical reaction.

precipitate
A solid that forms from a solution during a chemical reaction.

physical change
A change in a substance that does not change its identity

chemical change
A change in matter that produces one or more new substances

Evidence for a chemical change
production of gas (bubbles), formation of a precipitate, unexpected temperature change, color change, fire, a new product.

open system
A system in which matter can enter from or escape to the surroundings.
closed system
A system in which no matter is allowed to enter or leave
Law of Conservation of Mass
Matter is not created nor destroyed in any chemical or physical change
