ch 5: catecholamines
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
catecholamines are (2)
monoamines: a catechol + amine group
include dopamine, norepinephrine, epinephrine
catecholamine synthesis pathway (3)
synthesized by a multistep pathway in which tyrosine hydroxylase catalyzes the rate-limiting step
tyrosine → dopa → dopamine → norepinephrine
enzymes: tyrosine hydroxylase → aromatic amino acid decarboxylase (AADC) → dopamine ß-hydroxylase
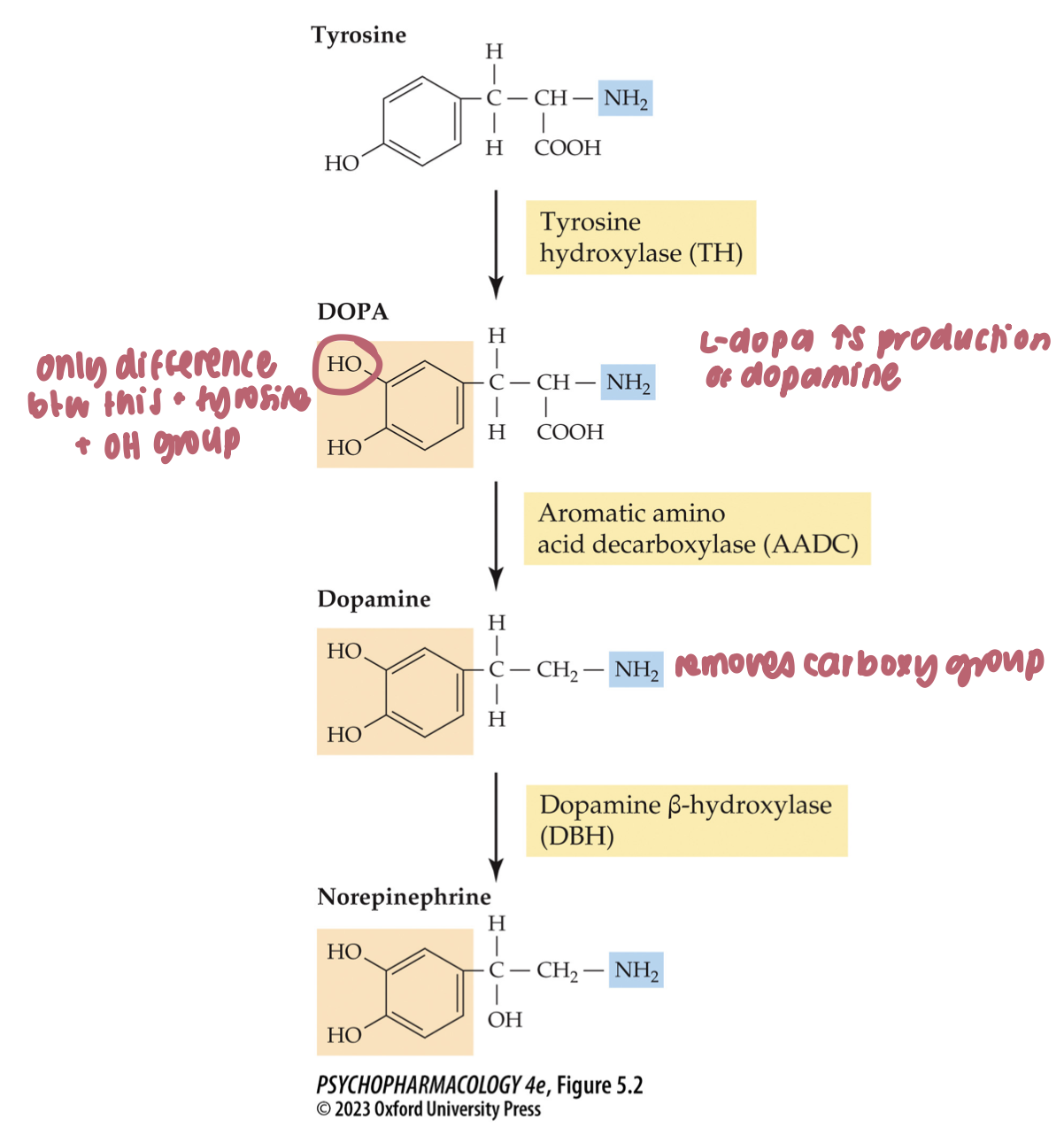
factors affecting tyrosine hydroxylase (TH) activity (2)
DA + NE lvls in the nerve terminal → negative feedback
cell firing stimulates TH activity through phosphorylation of the enzyme by protein kinases
how can catecholamine synthesis be increased/inhibited
↑sed: administering precursors → ie. tyrosine or L-DOPA
↓sed: inhibit one of the enzymes → α-methyl-para-tyrosine (AMPT) blocks TH
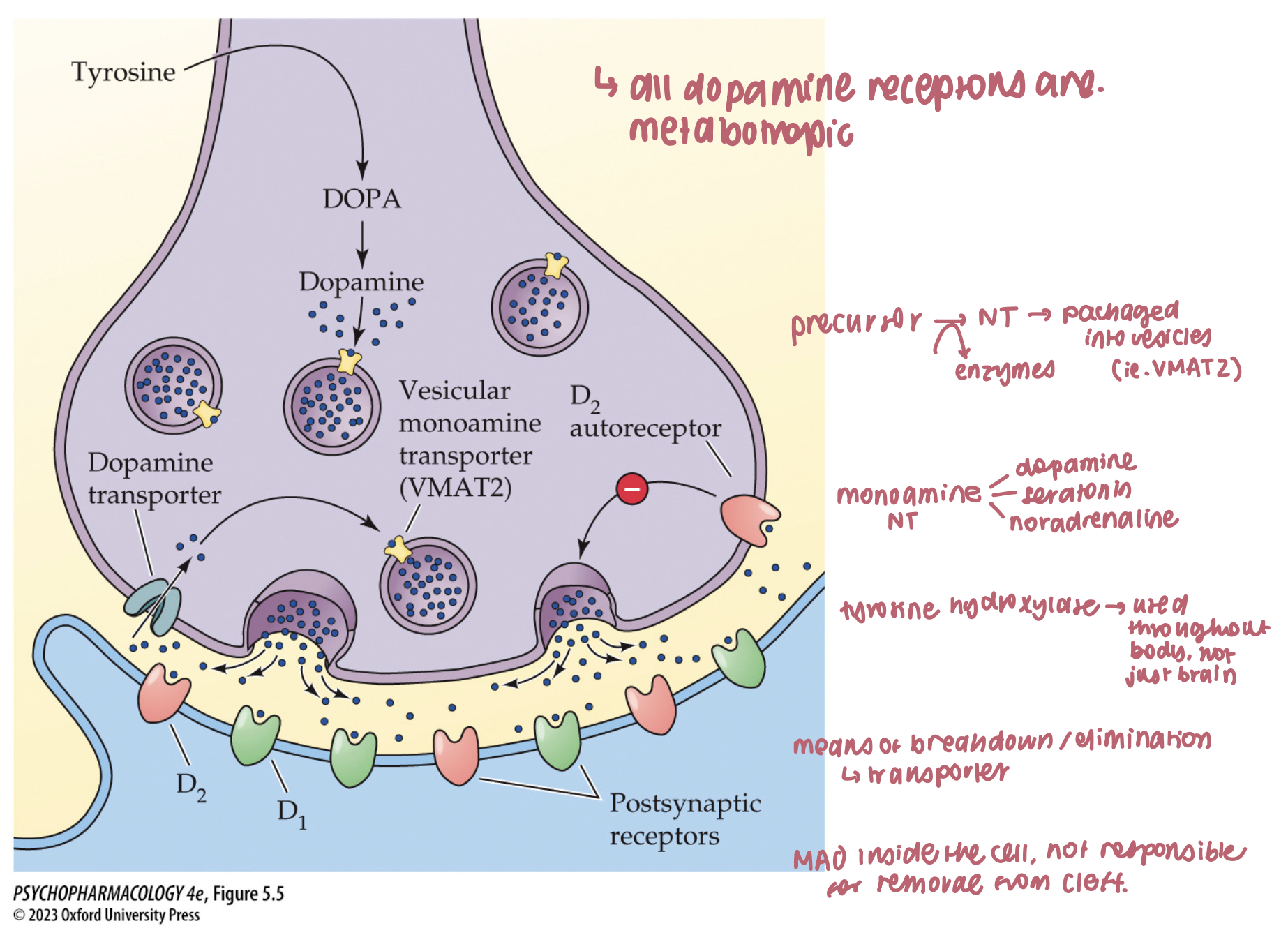
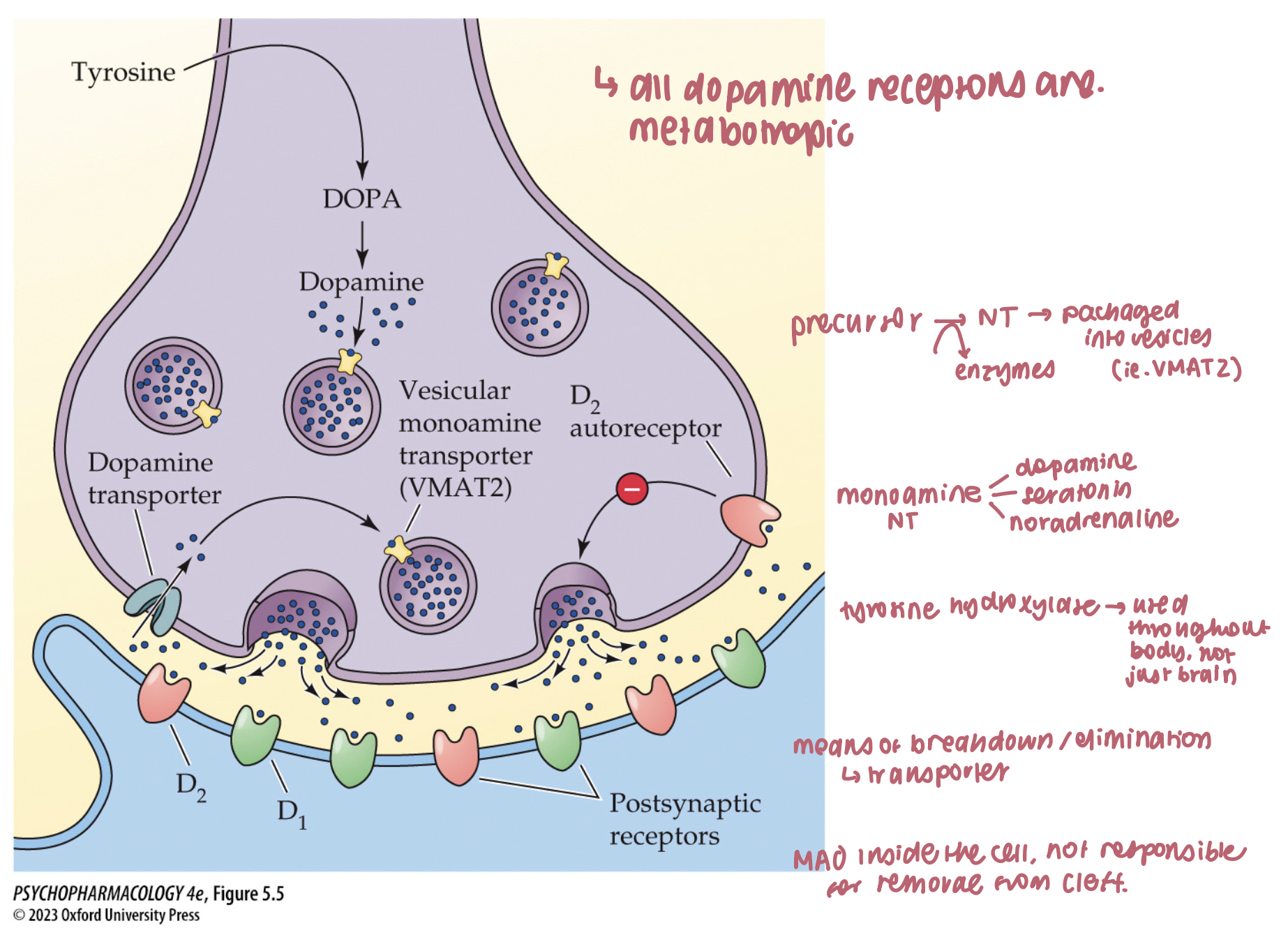
catecholamines are loaded into synaptic vesicles by ____ which can be blocked by _____ → an irreversible inhibitor that causes ____ and _____ symptoms
vesicular monoamine transporters (VMAT)
reserpine
sedation
depressive
reversible inhibitors of ____ are used to reduce uncontrolled movements in ____ disease + _____ dyskinesia
VMAT
Huntington’s
tardive (is a chronic, involuntary movement disorder that can develop as a side effect of taking certain medications)
How do amphetamine & methamphetamine affect catecholamine release, and what behaviors result? (3)
Mechanism (presynaptic): enter terminals via DAT/NET, displace NT from vesicles (VMAT2), and reverse transporters → release without nerve firing (action potential–independent).
Animals: ↑ general activity; high doses → stereotyped behaviors (repetitive sniffing/licking).
Humans: ↑ alertness, ↑ energy, euphoria, insomnia.
🧠 Takeaway: Psychostimulants can force DA/NE out even when the neuron isn’t firing—driving hyperactivity and, at high doses, stereotypies
What loads catecholamines into vesicles, and what happens if you block it? (3)
VMAT2 moves DA/NE into synaptic vesicles
Reserpine/tetrabenazine block VMAT → vesicular depletion, ↓ release
Classic effect: akinesia/depression-like behavior in animals; L-DOPA can rescue
🧠 Takeaway: No VMAT filling = no releasable DA/NE.
how does burst mode (phasic release) neuronal firing patter influence DA release compared to single-spiking mode? (tonic release)? (3)
burst mode: trains of 2-20 spikes at higher frequency
transmitter release occurring faster than it can be cleared and/or metabolized
enhanced release of transmitter + hangs around longer
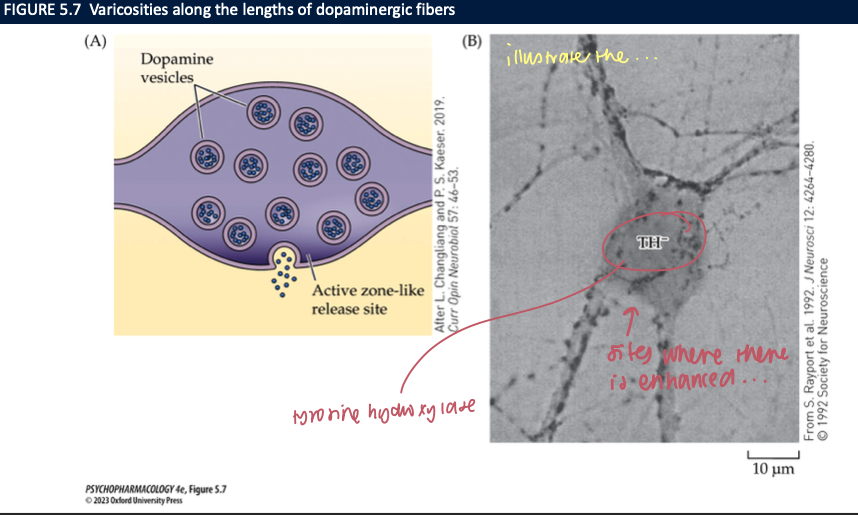
Where is DA released—classic synapses vs volume transmission (3)
Many DA axons have varicosities; only 30% show classic active-zone synapses
Much signaling is volume transmission: DA diffuses to extra-/perisynaptic receptors
Uptake by DAT/NET + local architecture sets the spread
🧠 Takeaway: DA often broadcasts via varicosities, not just tight synapses.
how are catecholamines recycled after release? + how can inhibitors ∆ this? (3)
DA + NE transporters return NTs to the releasing cell for breakdown or repackaging into vesicles
uptake by postsynaptic or glial cells
transporter-blocking drugs enhance DA/NE transmission by ↑ing the amount of NT available
nonselective vs selective MAO inhibitors (4)
non-selecetive: used to treat depression → had dangerous side effects
selective:
MAO-A: moclobemide: depression
MAO-B: selegiline (eldepryl) + rasagiline (azilect) → Parkinson’s disease
what two molecules are involved in the breakdown of catecholamines?
monoamine oxidase (MAO)
catechol-O-methyltrasnferase (COMT)
action of MAO and COMT produce what metabolites? what are these an indication of? (4)
DA metabolites: homovanillic acid (HVA)
NE metabolites:
3-methoxy-4hydroxy-phenylglycol (MHPG) in brain
vanillymandelic acid (VMA) in PNS
indication of catecholaminergic activity
what are the two important dopaminergic cell groups found in the midbrain? (4)
A9 → in substancia nigra → axons project to dorsal striatum in forebrain
A10 → in ventral tegmental area (VTA)
mesolimbic dopamine pathway
mesocortical dopamine pathway
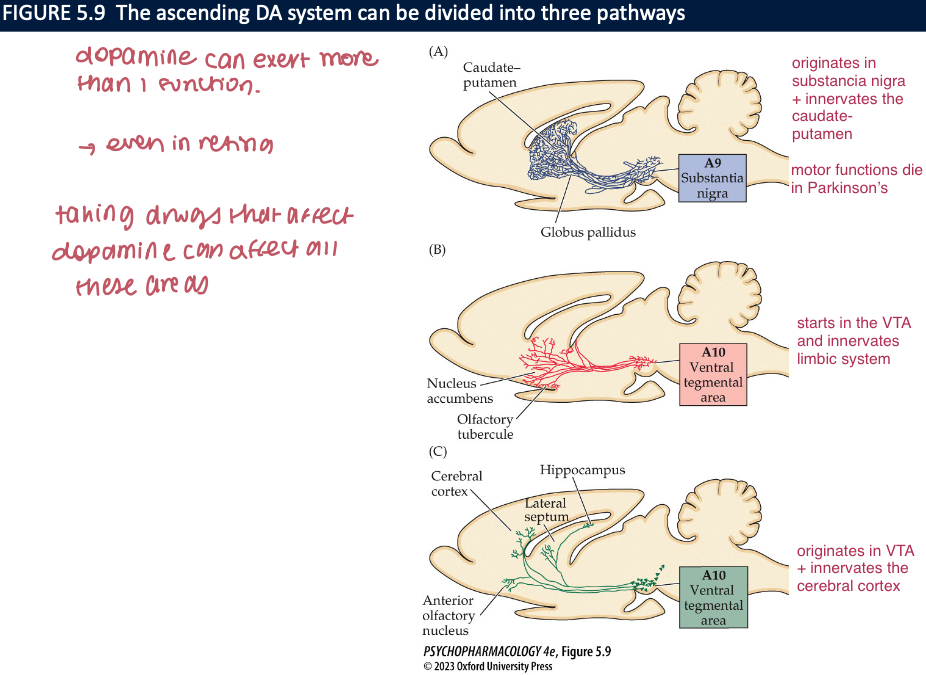
info about the motor functions of DA in humans has been derived from (4)
Parkinson’s disease
mutations in henes for TH, AADC, TH cofactor
experimental lesions of the nigrostriatal tract by neurotoxins that damage/destroy midbrain DA neurons + lesion their pathways
mice genetically engineered to lack DA → DD mice
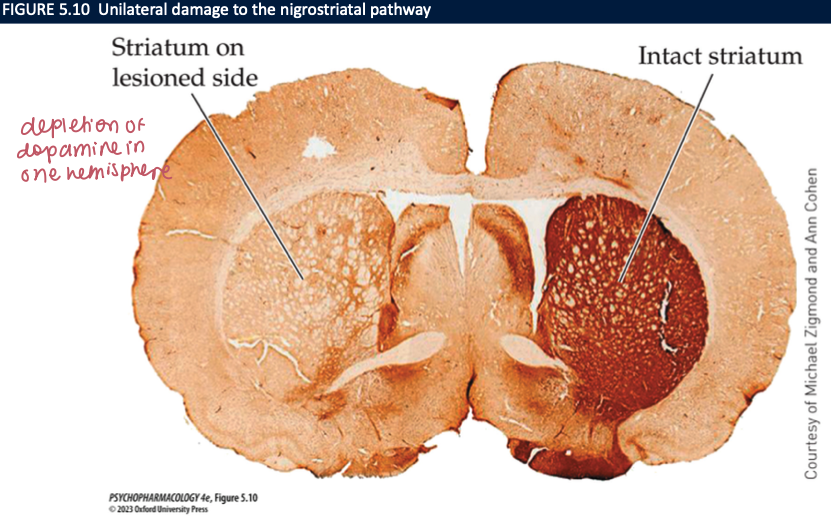
6-OHDA mice (3)
neurotoxin that is injected directly into the brain
causes severe damage and/or death to nerve terminals
animals result in sensory neglect, motivational deficits, motor impairments
how do DD mice differ to 6-OHDA mice? (3)
DA neurons undamaged → they just can’t make DA
DD mice lack DA throughout development
seem normal but after 1 week post birth: stop gaining weight, lack feeding + drinking behaviour, hypo-activity
what restores DD mice? (2)
temporarily: L-DOPA injection
long-term: restoring DA synthesis just in caudate-putamen
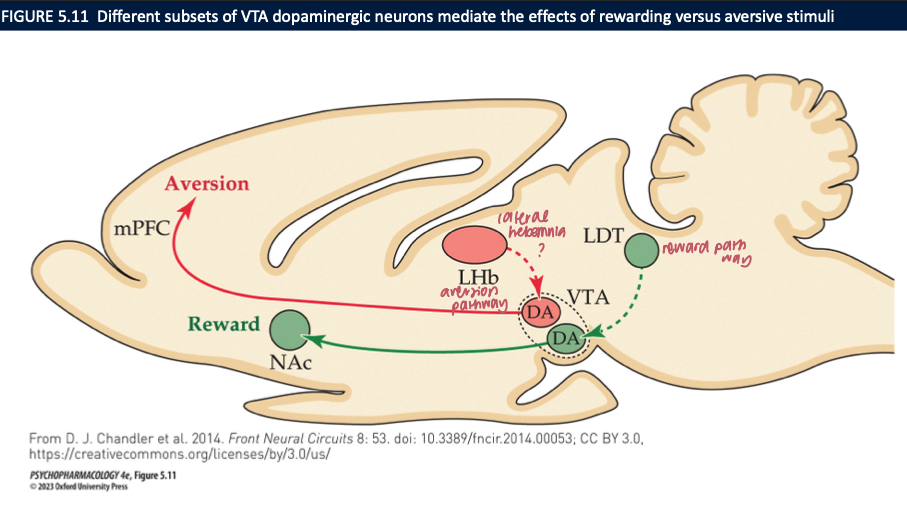
mesolimbic pathway activates _____ +______ behaviour → different neurons mediate the effects of ____ + ____ stimuli
arousal
locomotor
rewarding
aversive
mesocortical pathway: input to the ___, helps regulate cognitive functions ie. ____ and _____
PFC
attention
working memory
main subtypes of DA receptors (2)
all are metabotropic → 5 main subtypes
D1 and D2 are most common
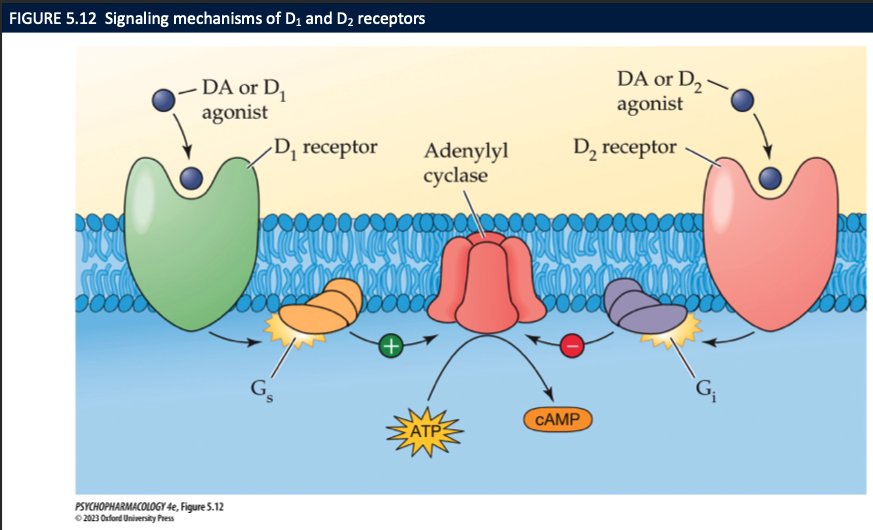
D1 vs D2 receptors (5)
D1 stimulate adenylyl cyclase → ↑ing rate of cAMP synthesis
D2 receptors inhibit adenylyl cyclase → ↓ing the rate of cAMP synthesis
also regulate membrane ion channels for K+
higher affinity for DA than D1
function as autoreceptors + postsynaptic receptors
hypothesis: tonic DA release activates higher-affinity D2 receptors resulting in
an increase in DA lvls produced by phasic release
what do dopamine receptor agonists typically do behaviorally? (3)
Increase locomotor activity and behavioral activation
Non-selective agonists (e.g., apomorphine) stimulate D₁ & D₂ receptors
Receptor-selective agonists help map which behaviors each subtype controls
give one D₁ agonist and one D₂/D₃ agonist used in research/clinic + what they’re used for (3)
D₁ agonist: SKF-38393 (research; receptor mapping; limited clinical use due to tolerance/tachyphylaxis)
D₂/D₃ agonists: bromocriptine / cabergoline / quinpirole
Uses: Parkinson’s (stimulate striatal DA), hyperprolactinemia (restore prolactin inhibition)
what do dopamine receptor antagonists do, and what happens at high doses? (3)
Block D₂ (± D₁) receptors → reduce dopaminergic behaviors
Antipsychotics (e.g., haloperidol) = D₂ blockers for schizophrenia
High doses → catalepsy / motor suppression (nigrostriatal D₂ block)
explain behavioral supersensitivity after chronic D₂ blockade (3)
Long-term D₂ antagonists (e.g., haloperidol) → up-regulation / increased sensitivity of postsynaptic D₂
After stopping the drug, animals show exaggerated responses to D₂ agonists
Mechanism base: receptor up-regulation in striatum
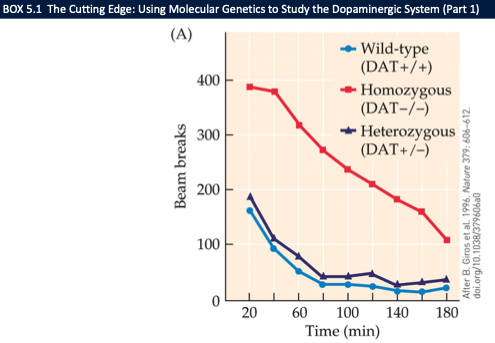
what do DAT knockout studies show about dopamine function? (3)
DAT −/− mice: hyperactive (persistent extracellular DA → ongoing receptor activation)
Show impulsivity/cognitive changes reminiscent of ADHD traits
Molecular genetics lets us knock in/out components to link DA pathways to behaviour
a non-selective dopamine agonist that activates both D₁ and D₂ receptors is _____, which typically _____ locomotor activity
apomorphine
increases
a research D₁ receptor agonist is _____; repeated dosing can lead to _____ (loss of effect)
SKF-38393
tachyphylaxis / tolerance
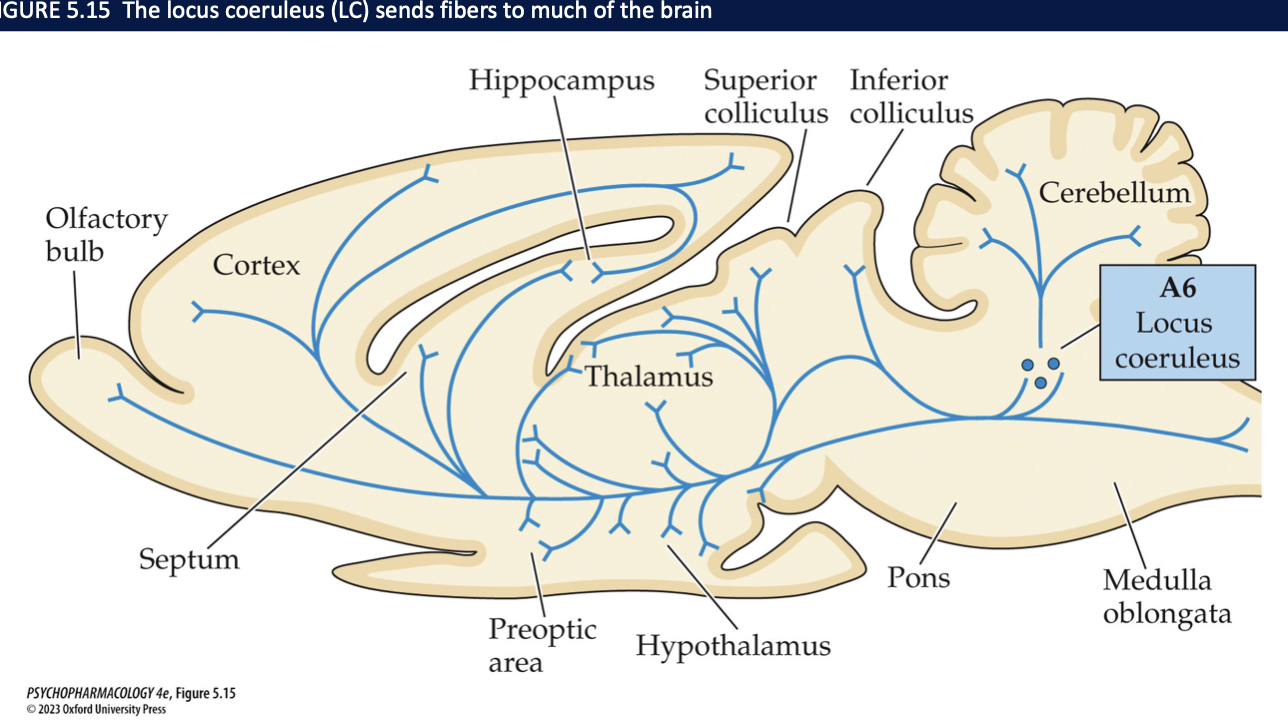
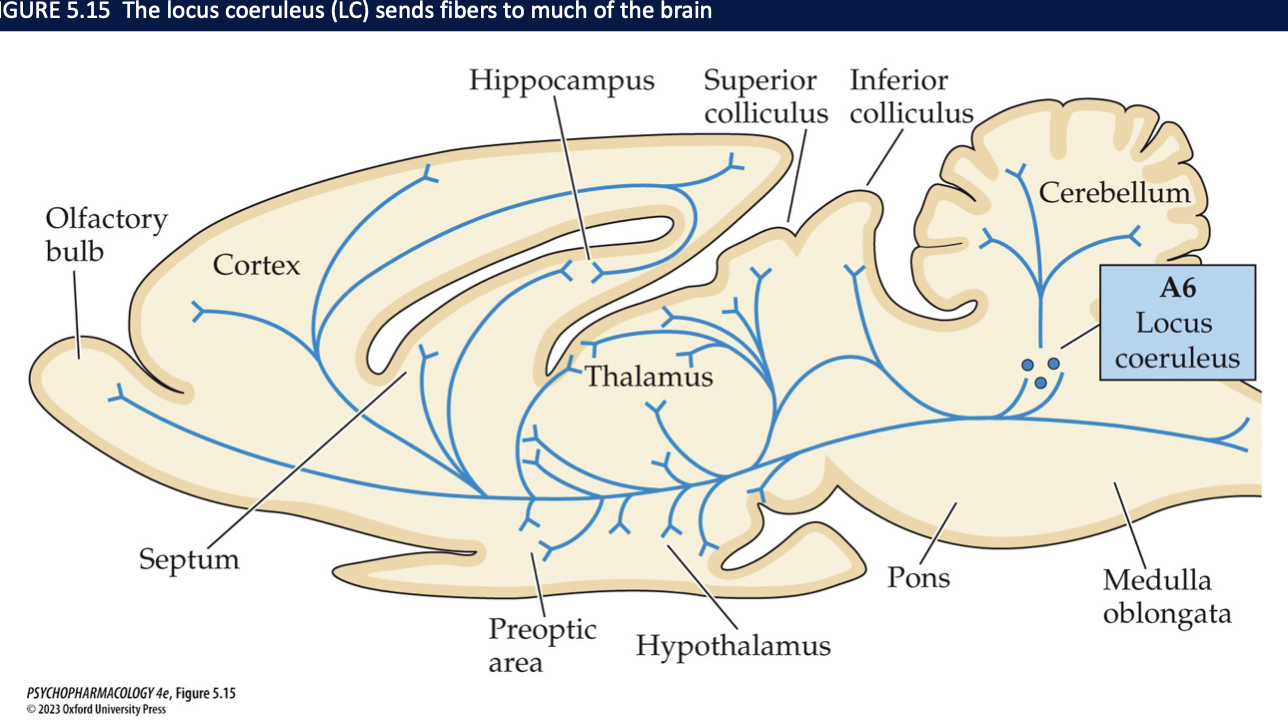
NE neurons are located in the ____ + medulla → especially in the ____. Here all neurons express the enzyme ____ + synthesize NE. This structure send fibers to ______ → areas mainly involved in _______ + cerebellum + spinal cord
pons
locus coerluleus (LC)
DBH
almost all areas of the forebrain
sensory information processing
D₂ receptor blockade by antipsychotics like _____ can cause _____ at high doses and may lead to _____ after chronic use
haloperidol
catalepsy / motor suppression
behavioral supersensitivity (D₂ up-regulation)
D₂/D₃ agonists such as _____ or _____ are used to treat _____ and _____ by stimulating dopamine receptors
bromocriptine
cabergoline (quinpirole = research)
Parkinson’s disease
hyperprolactinemia
deleting the dopamine transporter (DAT−/−) causes _____ behavior because extracellular DA _____
hyperactive/impulsive
stays elevated (reuptake impaired)
What do D1/D2/D3 KOs and DAT changes show about stimulant effects?
Receptor KOs blunt cocaine/amphetamine locomotion; DAT level (KO/over-expression) alters response → DA receptors + DAT are essential for stimulant behaviors.
Signaling for α1, α2, β1/β2/β3? (3)
α1: Gq ↑IP₃/DAG/Ca²⁺
α2: Gi ↓cAMP, opens K⁺ (often autoreceptor ↓ release)
β: Gs ↑cAMP.
How do α2 drugs change NE output & behavior? (2)
Clonidine/lofexidine (α2 agonists) ↓ LC firing → ease opioid withdrawal/HTN.
Yohimbine (α2 antagonist) ↑ NE release → anxiety/craving.
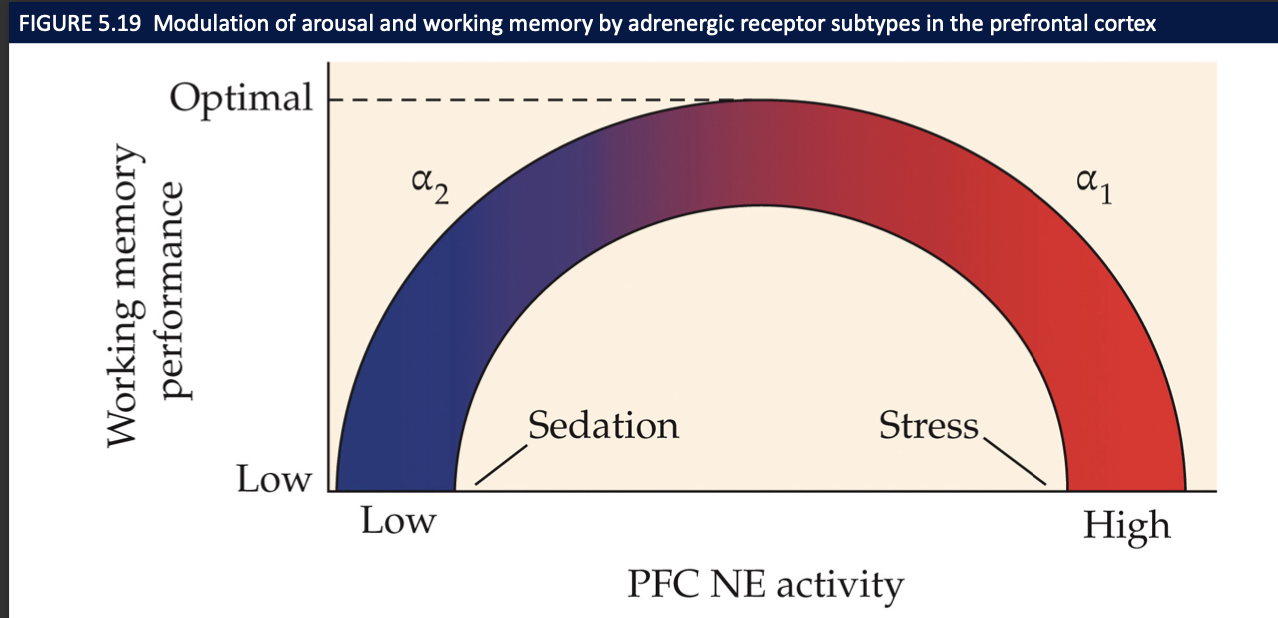
How does NE affect working memory in PFC?
Moderate NE via α2A → best WM; low NE (fatigue) and high NE (stress via α1/β) impair—classic inverted-U.
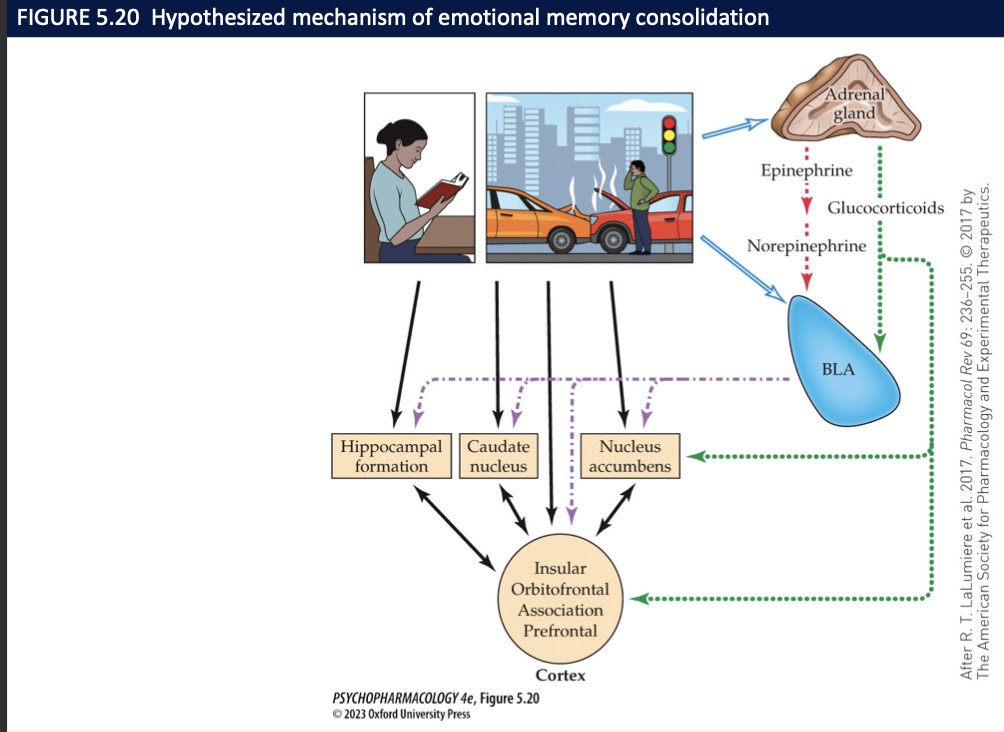
Why does stress strengthen emotional memories?
↑ LC NE + adrenal EPI/cortisol engages amygdala (BLA) → boosts hippocampus/PFC consolidation.
Key adrenergic agonist uses? (5)
β2 (albuterol/levalbuterol): bronchodilation (asthma/COPD).
Phenylephrine (α1): vasoconstriction/decongestant.
Isoproterenol (β1/β2): treats bradycardia.
Midodrine (α1): raises BP in orthostatic hypotension.
Dexmedetomidine (α2): ICU sedation/analgesia with minimal respiratory depression.
Key adrenergic antagonist uses? (3)
Prazosin (α1 block): HTN, PTSD nightmares.
Propranolol (β1/β2): ↓ HR, performance anxiety;
Metoprolol (β1-selective): cardiac control with fewer bronchospasm risks.