Kinetics
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
what is kinetics
study of reaction rates
What must happen for a reaction to occur
molecules must collide
what must happen to have successful collisions
must produce enough energy to produce a reaction
Has to have the correct orientation
what are the ways to increase the number of collisions
Increase concentration-more reactants to combine
Increase temp-increase in kinetic energy
Use a catalyst-lowers activation energy
what is thermochemistry
study of heat absorbed or released in a chemical equation
What happens with heat in endothermic reactions
absorbed- makes container feel cool
What happens with heat in exothermic reactions
released- makes container feel warm
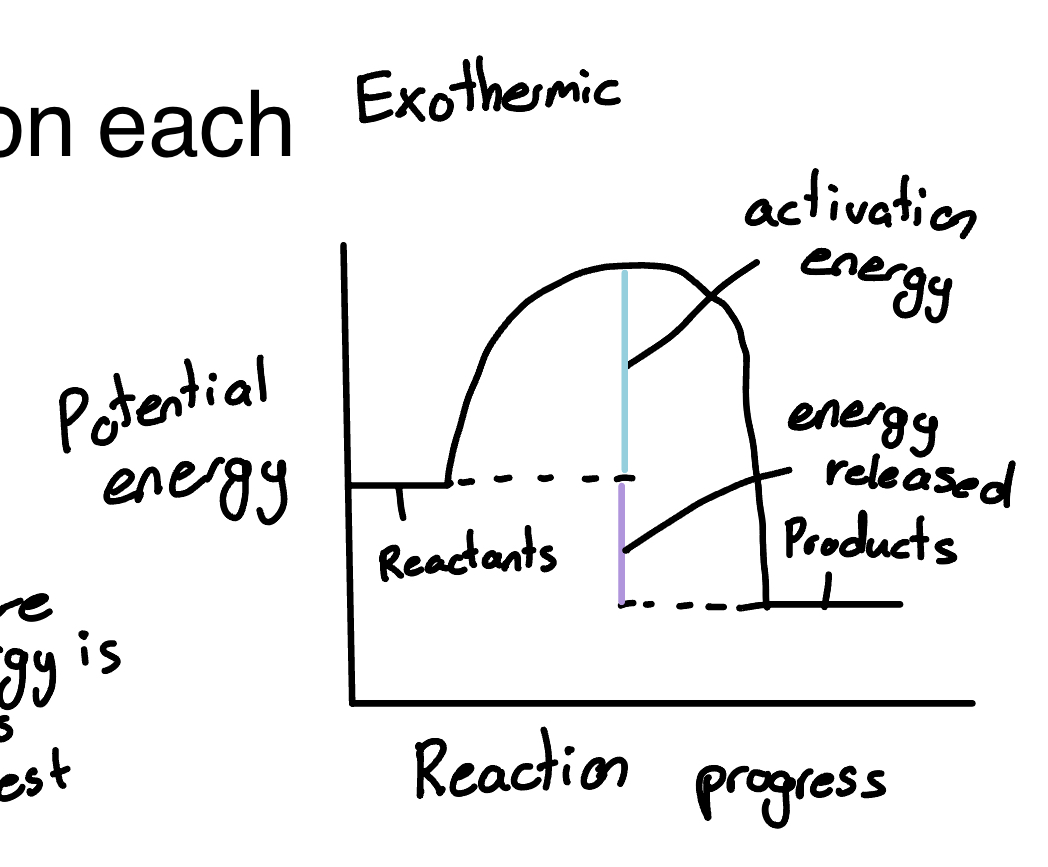
Draw the exothermic reaction
Draw the endothermic reactions
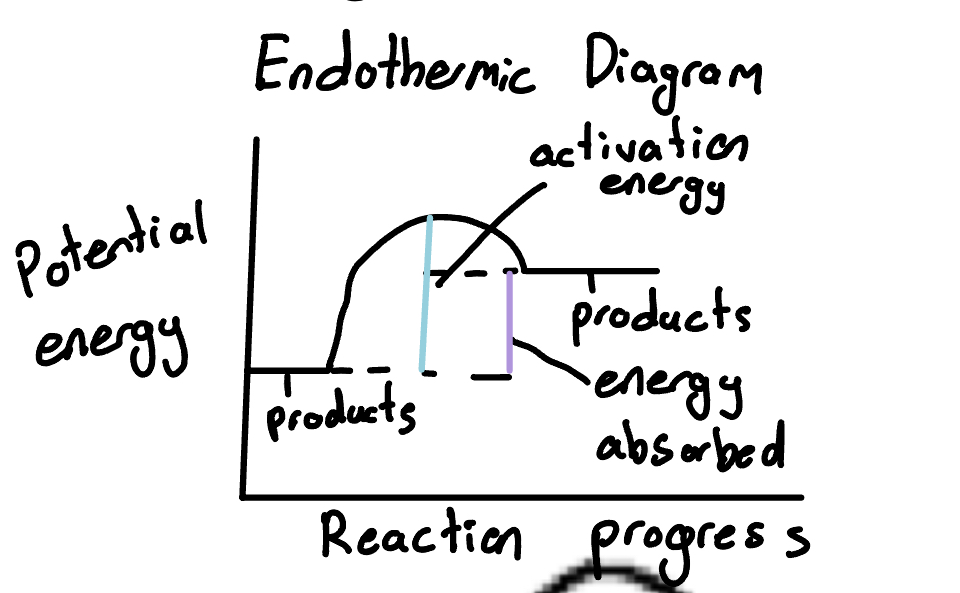
What is activation energy
the amount of energy that must be overcome before reaction can occur
Activated complex
where energy is at its highest
Highest point on mountain part of graph
What are the 2 units of energy
calorie and joule
What is the symbol for enthalpy
triangle H
Pronounced delta H
What is enthalpy
the measures the heat content of a reaction at a constant pressure
Do endothermic reactions have a positive or negative enthalpy
positive
Do exothermic reaction have a positive or negative enthalpy
negative
If delta H is on reaction side what kind of reaction is it
endothermic
If delta H is on product side what reaction is it
exothermic