Lecture 31 and 32- Valance Bond Theory, Sigma and Pi Bonding, isomerization
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Once you know the electron-domain geometry, you know the hybridization state of the atom. True or False?
True.
An "s" and "p" orbital are combined to create ___(amt) _____(type) hybrid orbitals which are seperated by 180 degrees.
1) one
2)sp
Just like linear geometries
Are the sp2 orbitals located in the same plane? What are they composed of?
1) Yes, 120 degrees apart
2) composed of:
1s+2p= 3sp^2 orbitals
Which hybridized orbitals make up a tetrahedral geometry?
1) sp^3 orbitals
S orbitals+ 3 P orbitals= 4 hybrid orbitals
seperated by 109.5 degrees
sp^3d makes up _________ geometry.
trigonal bipyramidal
sp^3d^2 makes up _________ geometry.
octahedral
The atoms are in the same plane when hybridized unitl the Pz orbital is used. Explain?
There is three P orbitals. Px, Py, and Pz. Pz makes it 3D, so unless that orbital is used the molecule will be planar.
*The Pz orbital is the last one used*
What are Pi bonds characterized by:
Pi bonds are characterized by:
Side-to-side overlap
Electron density above and below the internuclear axis
Are single bonds Pi or sigma? Why
Single bonds are always sigma bonds, because simag overlap is greater, resulting in a stronger bond and lower energy
If a molecule has 3 bonds what type would they be? and how many of that type?
In a multiple bond one of the bonds is a sigma bond and the rest are pi bonds
Pi bonds occur in ______ P orbitals.
unhybridized
In reality, each of the four atoms in the nitrate ion has a p orbital. The p orbitals on all three oxygens overlap with the p orbital on the central nitrogen
**For NO3
This means the pi electrons are not localized between the nitrogen and one of the oxygens, but rather are delocalized throughout the ion
Can Pi bonds be twisted? If not, which type of bonds can?
For Pi bonding, the unhybridized p orbitals of the two atoms must be aligned for proper overlap
Rotation would diminish this overlap
A twist of 90 degrees would break this bond (requires about 260 kJ/mol)
Why do we use Molecular Orbital theory rather than Valance bond theory??
Though valence bond theory effectively conveys most observed properties of ions and molecules, there are some concepts better represented by molecular orbitals
For example, valence bond theory does not correctly predict the electron structure of O2 (paramagnetic)
What are the 4 basic principles for the molecular orbital theory?
1) The total number of molecular orbitals is always equal to the total number of atomic orbitals contributed by the atoms that have combined
2)The bonding molecular orbital is lower in energy than the parent orbitals, and the antibonding orbital is higher in energy
3)Electrons of the molecule are assigned to orbitals of successively higher energy according to the Pauli exclusion principle and Hund's rule
4)Atomic orbitals combine to form molecular orbitals most effectively when the atomic orbitals are of similar energy
What does it mean when they say that orbitals have wave like characteristics?
In MO theory, we invoke the wave nature of electrons
If waves interact constructively, the resulting orbital is lower in energy: a bonding molecular orbital
If waves interact destructively, the resulting orbital is higher in energy: an antibonding molecular orbital
How do you find the Bond order?
The bond order is one half the difference between the number of bonding and antibonding electrons
Which period of elements is different from all the other ones when it comes to MO theory?
The smaller p-block elements in the second period have a sizeable interaction between the s and p orbitals
This flips the order of the sigma and pi molecular orbitals in these elements
what is the order that the bonds are put in for MO diagrams?
What is the exception?
Sigma* 2P
Pi* 2P
Pi 2P
Sigma 2P
Sigma 1s*
Sigma 1s
2nd row of elements:
Sigma* 2P
Pi* 2P
Sigma 2P
Pi 2P
Sigma*2s
sigma 2s
Sigma BOnd
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond.[1] They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is symmetrical with respect to rotation about the bond ax
Pi Bonds
pi bonds (π bonds) are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital. These orbitals share a nodal plane which passes through both of the involved nuclei.
Atomic Orbital
A mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom.[1] This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region where the electron can be calculated to be, as defined by the particular mathematical form of the orbita
How are orbital in an atom is characterized
by a unique set of values of the three quantum numbers n, l, and m, which correspond to the electron's energy, angular momentum, and 3 Quantum Numbers (n represents). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number l = 0, 1, 2 and 3 respectivel
3 Quantum Numbers (n represents)
the electron's energy, (n)
3 Quantum Numbers (l represents)
the electron's angular momentum (l)
s-orbital
s orbital refers to an orbital with angular momentum quantum number l=0
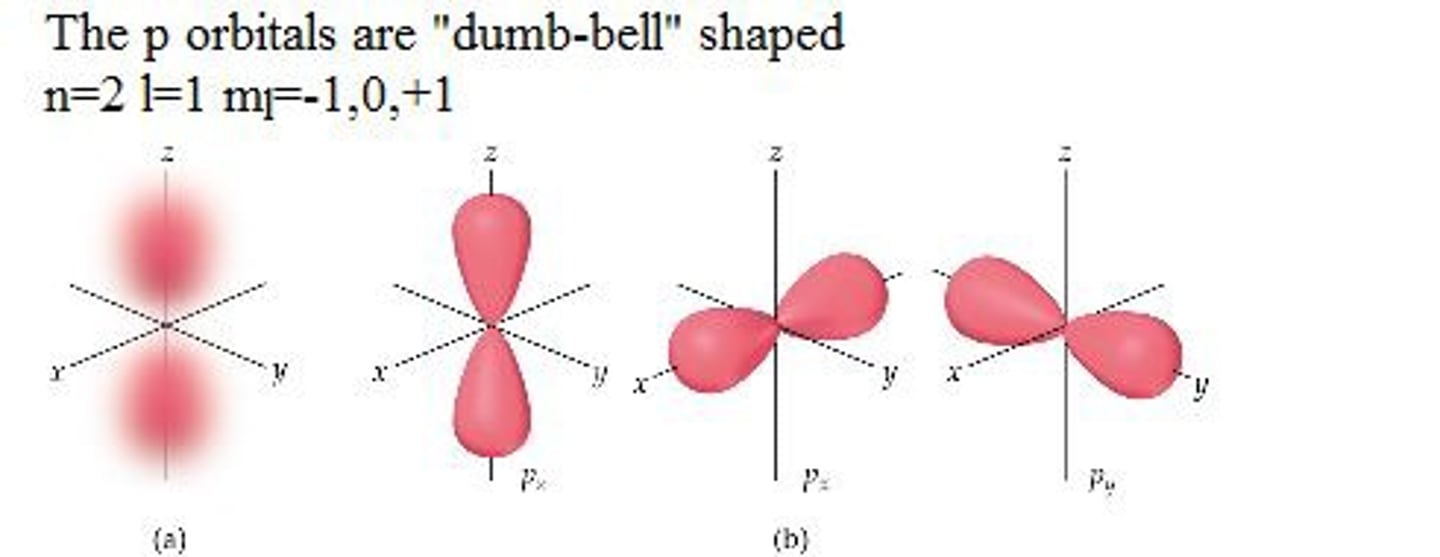
p- orbital
p orbital refers to an orbital with angular momentum quantum number l=1
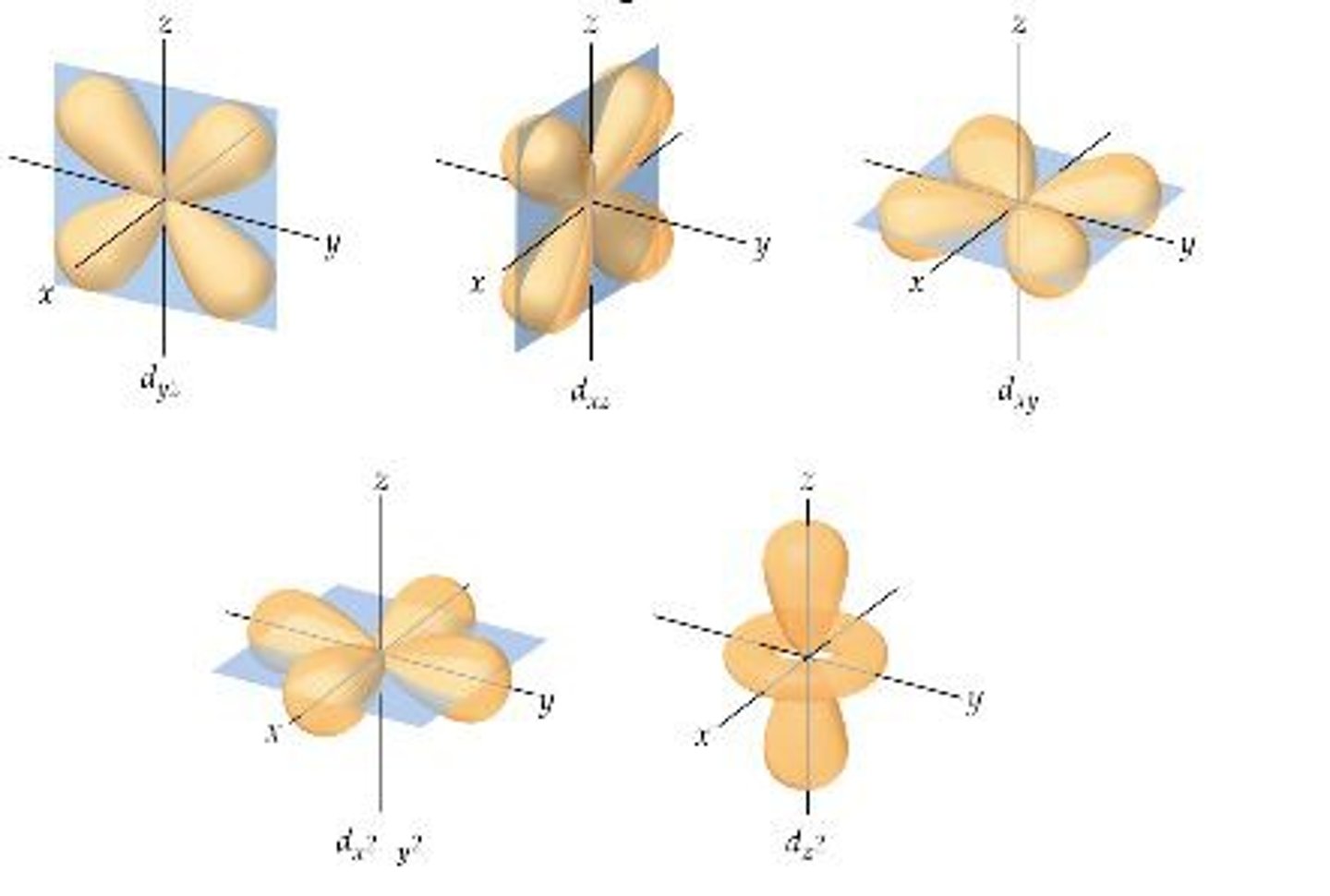
d-orbital
d orbital refers to an orbital with angular momentum quantum number l=2
f-orbital
f orbital refers to an orbital with angular momentum quantum number l=3
molecular orbital diagram, or MO diagram
a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that AS ATOMS BOND TO FORM MOLECULES, A CERTAIN NUMBER OF ATOMIC ORBITALS COMBINE TO FORM THE SAME NUMBER OF MOLECULAR ORBITALS , although the electrons involved may be redistributed among the orbitals
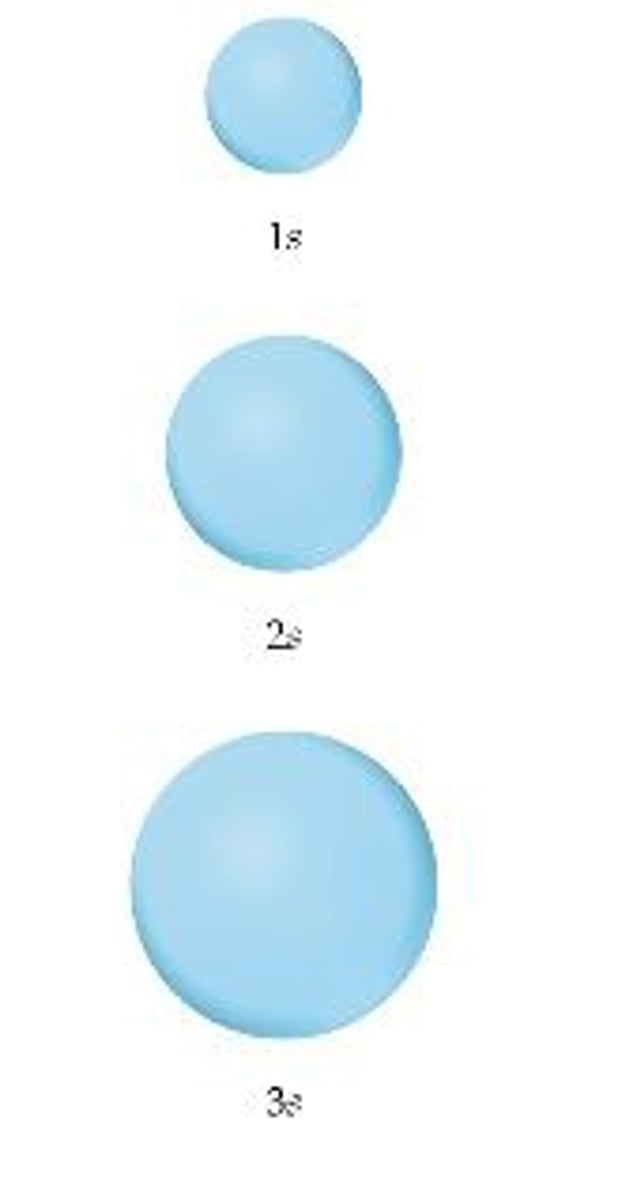
s-orbital
s orbitals are spherical in shape
The 1s orbital has n=1 l=0 and ml = 0
The 2s orbital has n=2 l=0 and ml = 0
The 2s orbital has n=3 l=0 and ml = 0
d orbital
p orbital
Which orbital is more effective in screening?
1s or 2p?
1s
Penetration
Penetration describes the proximity of electrons in an orbital to the nucleus. Electrons which experience greater penetration experience less shielding and therefore experience a larger Effective Nuclear Charge (Zeff) but shield other electrons more effectively. Electrons in different orbitals have different wavefunctions and therefore different distributions around the nucleus.
Orbital Penetration
Different orbitals have greater nuclear penetration than others. Penetration refers to how effectively electrons can get close to the nucleus. The electron probability density for s-orbitals is highest in the center of the orbital, or at the nucleus. If we imagine a dartboard that represents the circular shape of the s-orbital and if the darts landed in correlation to the probability to where and electron would be found, the greatest dart density would be at the 50 points region but most of the darts would be at the 30 point region. When considering the 1s-orbital, the spherical shell of 53pm is represented by the 30 point ring.
Shielding
Shielding describes the amount of screening from nuclear charge that one electron can do with respect to its neighboring electrons. Electrons that have greater penetration can get closer to the nucleus and effectively block out the charge from electrons that have less proximity. For example, Zeff is calculated by subtracting the magnitude of shielding from the total nuclear charge. The value of Zeff will provide information on how much of a charge an electron actually experiences.
Radial Distribution Graphs
A radial distribution function graph describes the distribution of orbitals with the effects of shielding.
The greater the penetration of an orbital, the greater the screening capability of that orbital.
TRUE: The greater the penetration of an orbital, the greater the screening capability of that orbital.
Find the Zeff of
a.Mg
b. C
c. F
d. Ca
a. 12-2 =10
b. 6-4=2
c. 9-7=2
d. 20-2=18
Which of these have the smallest electron affinity? B, C, N, O, or F.
B
Periodic Trends Due to Penetration and Shielding
EFFECTIVE NUCLEAR CHARGE(Zeff)
The effective nuclear charge increases from left to right and increases from top to bottom on the periodic table.
Shielding
Shielding describes the amount of screening from nuclear charge that one electron can do with respect to its neighboring electrons. Electrons that have greater penetration can get closer to the nucleus and effectively block out the charge from electrons that have less proximity.
Periodic Trends Due to Penetration and Shielding: ELECTRONEGATIVITY
The electronegativity of the elements is highest near flourine. In general, it increases from left to right and decreases from top to bottom.
Periodic Trends Due to Penetration and Shielding: IONIZATION ENERGIES
The ionization energies increase from left to right, and decrease from top to bottom.
Periodic Trends Due to Penetration and Shielding: ATOMIC RADIUS
The atomic radius decreases from left to right, and increases from top to bottom.
Hybrid orbitals allows us to use
valence bond theory to describe covalent bonds (sharing of electrons in overlapping orbitals of two atoms)
http://www.mikeblaber.org/oldwine/chm1045/notes/Geometry/Hybrid/Geom05.htm
sp orbital
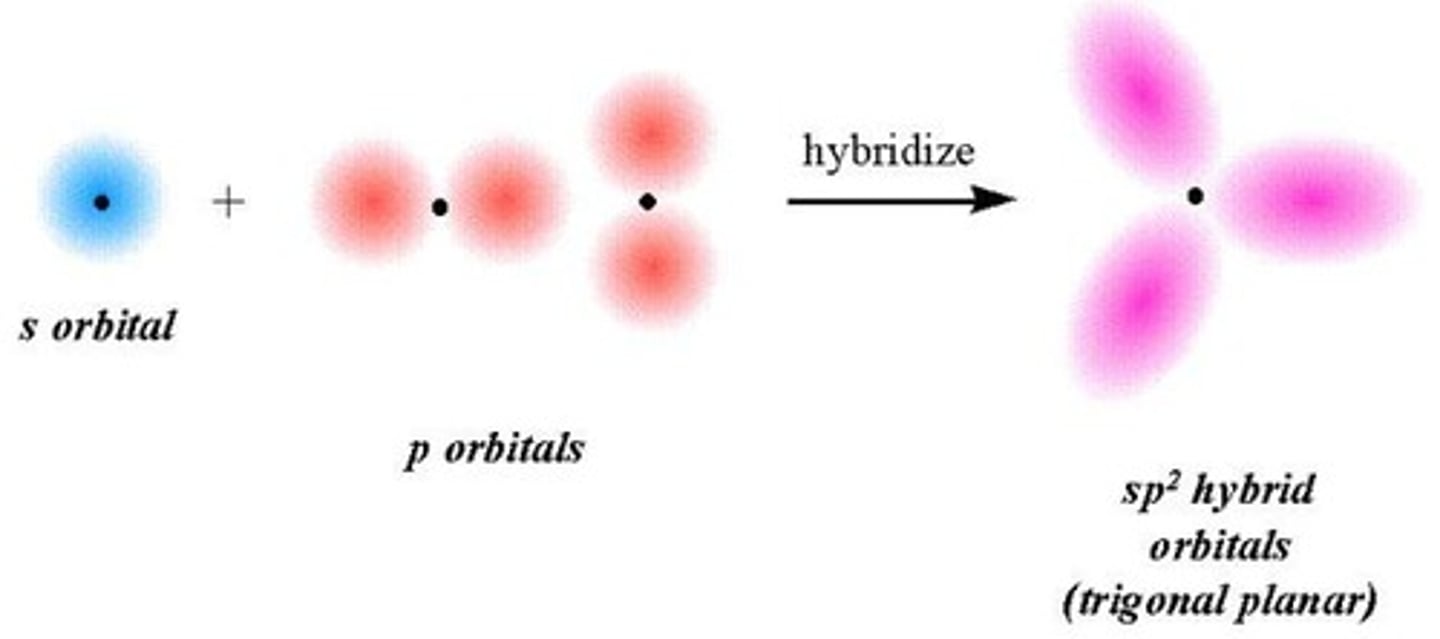
When we know the molecular geometry, we can use the concept of hybridization
to describe the electronic orbitals used by the central atom in bonding
3 Steps Steps in predicting the hybrid orbitals used by an atom in bonding:
1. Draw the Lewis structure
2. Determine the electron pair geometry using the VSEPR model
3. Specify the hybrid orbitals needed to accommodate the electron pairs in the geometric arrangement
NH3
1. Lewis structure
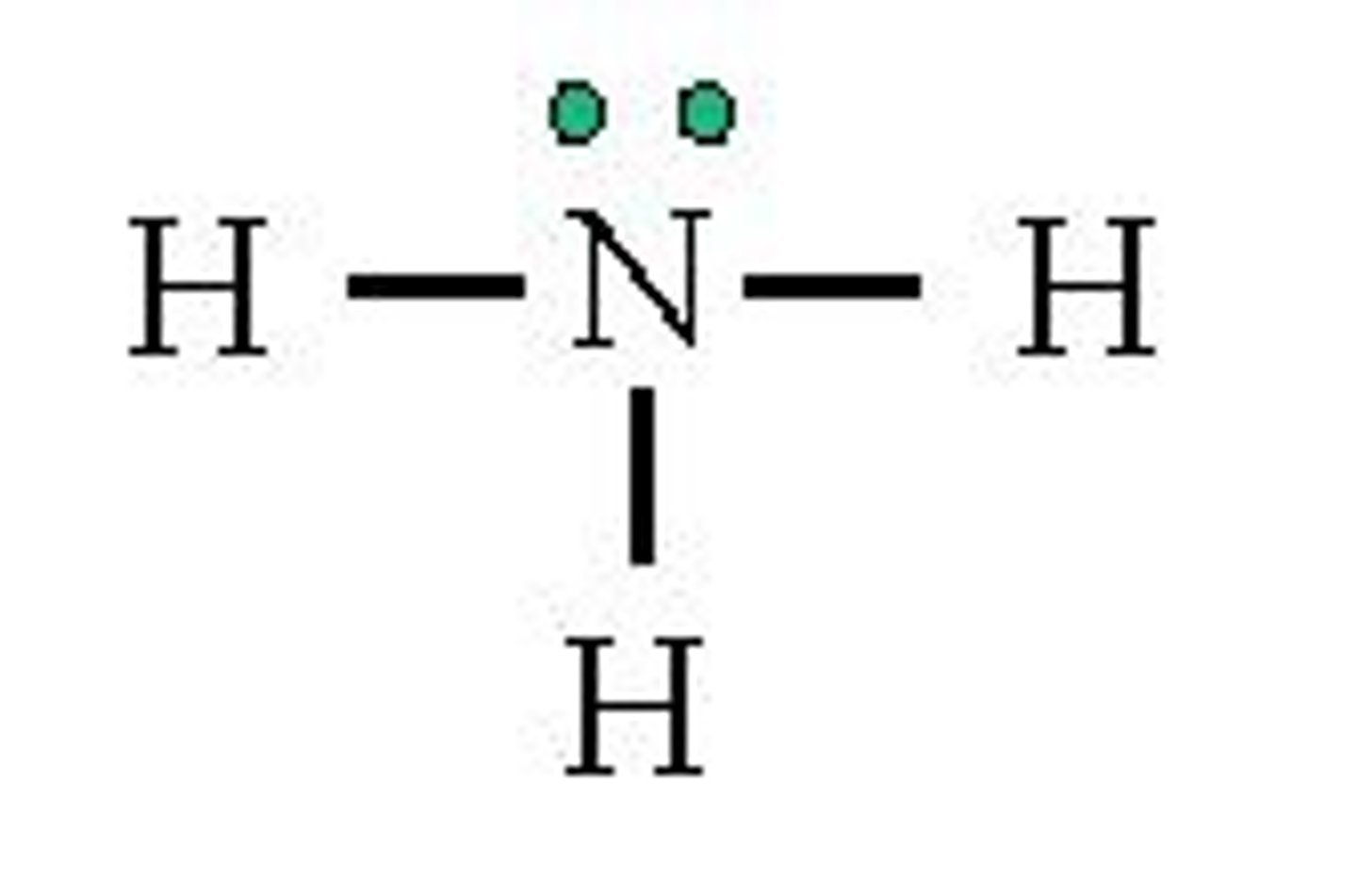
NH3 VSEPR indicates
VSEPR indicates: tetrahedral geometry with one non-bonding pair of electrons (structure itself will be trigonal pyramidal)
Rule for Hybridization
rule for hybridization:
# of hybridized orbitals =
#of attached atoms + # of lone pairs
Trigonal Pyramidal. Hybridization: sp3