Functional group tests and solubility rules
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Test for alkenes (2)
1. Decolourises orange bromine water when shaken at room temp
2. Add acidified potassium manganate (VIII) that goes from purple-brown to colourless
Test for alcohol (-OH or -COOH)
Phosphorus (V) pentachloride at room temperature
Steamy white fumes that turn damp blue litmus paper red (HCl)
Test for aldehyde or ketone (C = O)
Ad 2,4-DNPH/Brady's reagent at room temperature
Yellow or orange ppt
Test for aldehyde (-CHO) (2)
1. Add Fehling's solution and warm. Changes from blue to green and a red ppt forms
2. Add Tollen's reagent. Forms a silver mirror or dark grey solid.
Test for a primary or secondary alcohol, or an aldehyde
Add acidified potassium dichromate. Changes from orange to green.
Test for a methyl ketone or ethanal
Add iodine solution (iodoform) and warm in NaOH.
Pale yellow ppt forms which has an antiseptic smell.
Test for carboxylic acid (-COOH only) (CO2 is produced)
Add sodium carbonate at room temperature.
Effervescence and CO2 is produced which when bubbled through limewater, will turn it from colourless to cloudy.
Test for carboxylic acid (-COOH only) (Ester is produced)
Add ethanol/any alcohol to form an ester. Pour it into sodium carbonate solution and warm with sulphuric acid
Fruity smell
All common sodium, potassium, and ammonium salts (e.g. their carbonates and hydroxides) are
soluble
all nitrates are
soluble
most common chlorides and sulphates are
soluble
carbonates, phosphates, and sulphides are
insoluble, except those containing alkali metals and ammonia
Test for haloalkanes
Add NaOH and warm in a water bath.
Then acidify with HNO3 followed by AgNO3
Cl - white ppt
Br - cream ppt
I - yellow ppt
Test for alcohol (-OH only)
Add ethanoic acid
then sulphuric acid and sodium carbonate (SES)
An ester is formed which has a fruity smell
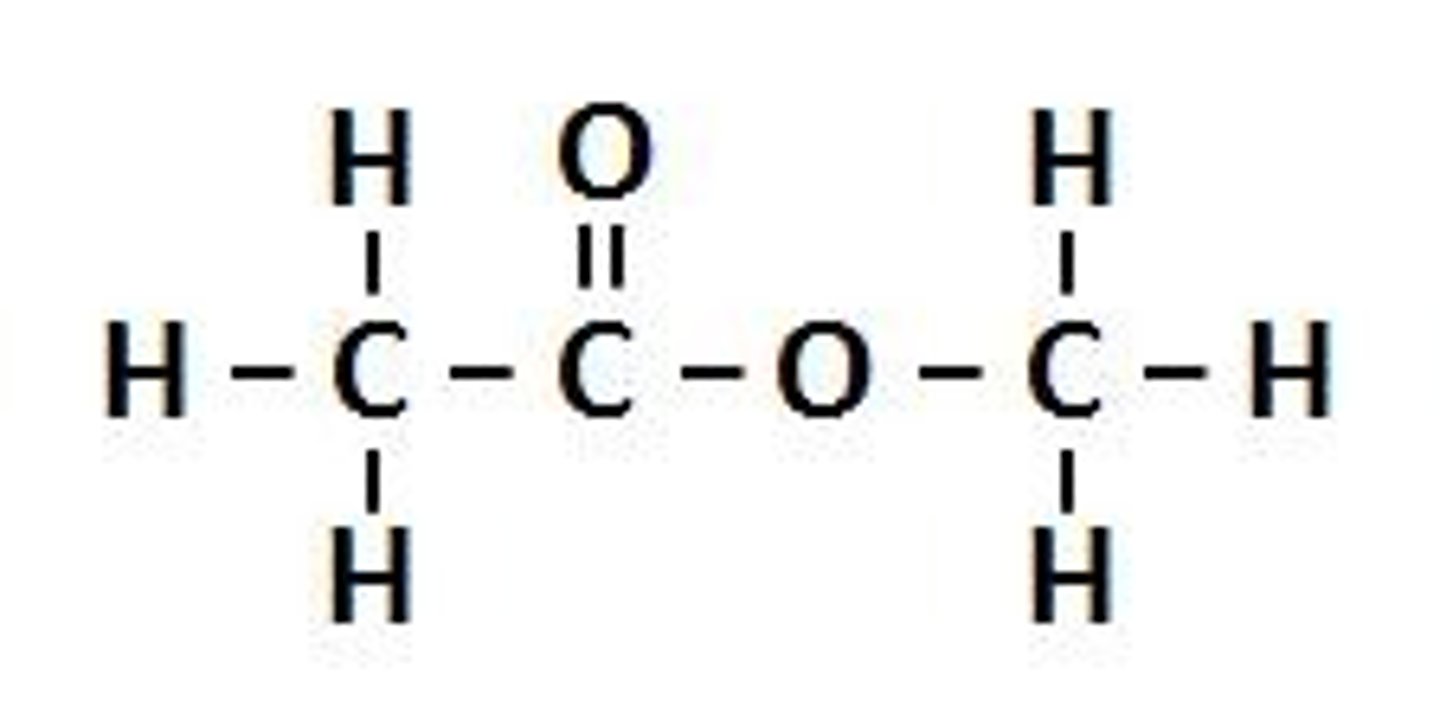
silver and lead chloride are
insoluble
lead and barium sulphate are
insoluble
Still learning (12)
You've begun learning these terms. Keep up the good work!