Trace Evidence
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
DNA does not have all answers…
- Not all crime scene having recoverable DNA.
- Secondary transfer of DNA, thus unreliable.
- Lack of database matches.
- Contamination
Transfer considerations
- Nature of trace
- Shedding
- Substrate and affinity
- Nature of contact
- Frequency
- Surface area
- Timeframe – how long has the material been there, is it fresh or been there a long time?
Persistence: continued or prolonged existence of something
Persistence considerations + concerns
- Nature of trace, so same considerations as transfer ones.
However, other considerations required:
- Emergency services intervention
- Contamination
- Post-deposition activity
- Cleaning/laundering
Concerns:
- Environmental
- Weathering
- Animals
- Microorganisms
- Crime à recovery time
- Recovery à analysis time
- Trace embedding
Trace evidence challenges
- Efforts of emergency personnel
- Scene tampering
- Not detected
- Detected but value not recognised
- Detected and recognised but not relevant to case
- Overwhelming amount of trace – anything can be evidence
- Inappropriate collection technique – loss/degradation
- Incorrect analytical technique
- Inaccurate interpretation
Recovery considerations
- Recover trace or entire item?
- Gloved hand or tweezers?
- Wet samples?
- Gelatine lifters
- Sequential vs zonal tape lifting
- Vacuum sweeping
- Swabbing
- Control samples
- ESLA – not just for footwear
- Hinge lifters – not just for finger marks
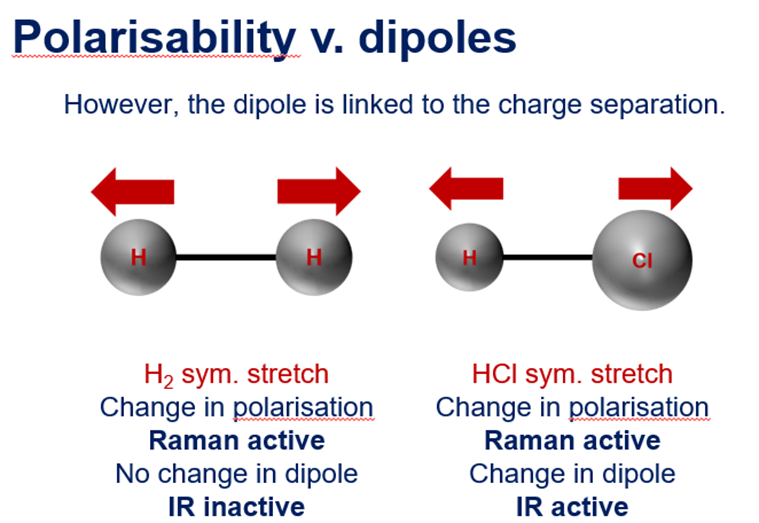
Raman vs IR
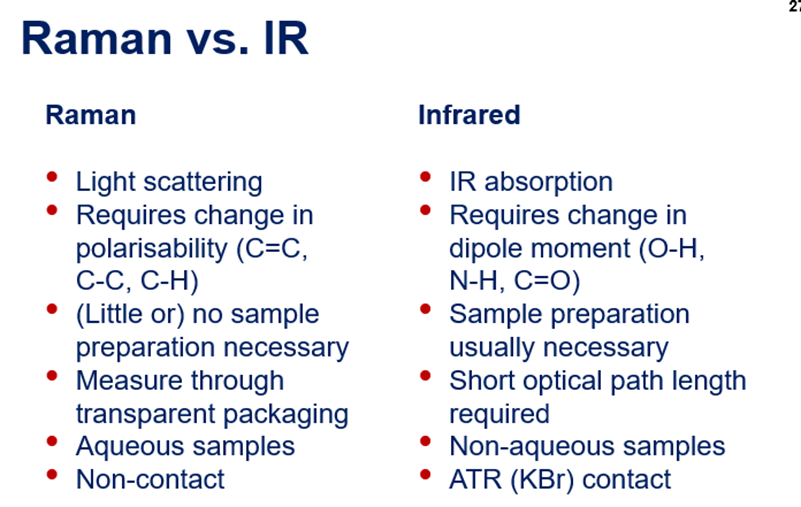
Raman - beware of heterogeneity – if a sample is not fully homogeneous, and care needs to be taken, when data is collected from point spectra.
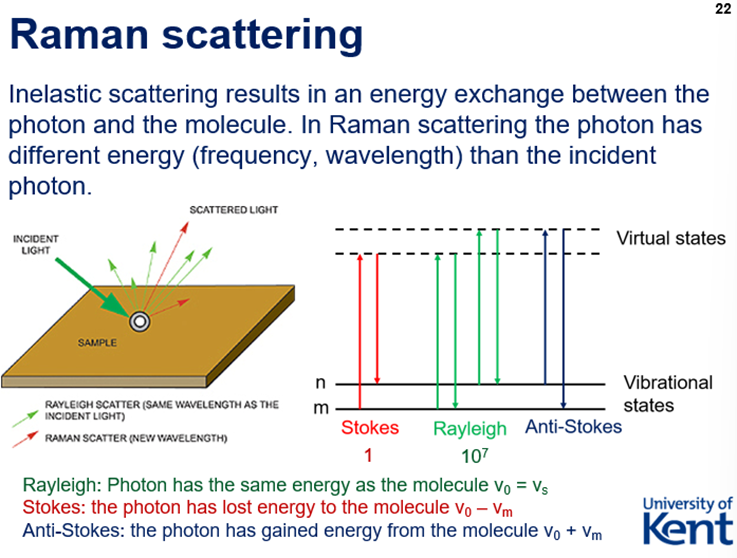
Photon scattering
Photons interact with molecular vibrations in a material and are scattered.
Elastic = leaves the molecule in the same state – Rayleigh scattering
Inelastic = leaves the molecule in a different quantum state – Raman scattering
Uses of Raman Spectroscopy
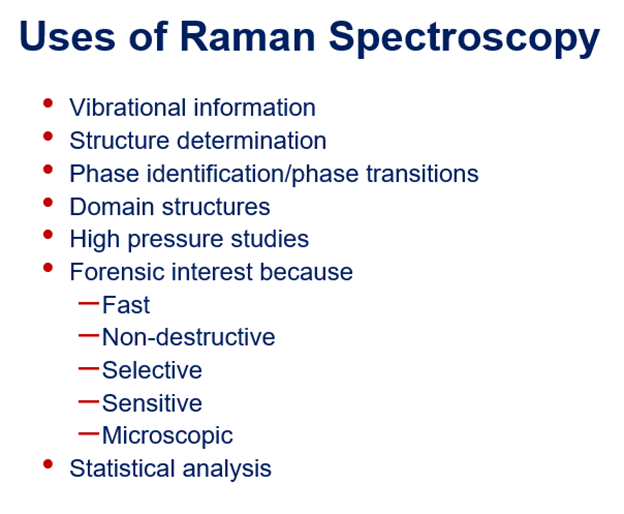
Forgery detection, as Raman spectra is a measure of particular vibrations, it is unique to a particular molecule/material. E.g., fake diamonds, jewellery.
Foreign substances in fingerprints
Wavelength + frequency definitions
1 wavelength = the distance from a point in a cycle to the corresponding point in the next cycle.
Longer wavelengths vibrate fewer times so the longer the wavelength, the lower the frequency.
Frequency = the number of vibrations of a given wavelength in one second, measured in hertz (Hz).
1 Hz = 1 wave completed per second.
In vacuum, all waves in the electromagnetic spectrum travel at the same speed = 3 x 108 ms-1
Properties of waves related by formula: velocity (c) = frequency (f) x wavelength (lambda)
Absorption, reflection + refraction (+ factors affecting) definitions
Absorption: when a photon of light enters a material but does not exit again. Results in thermal, electrical, or chemical changes.
Reflection: light ray is turned back into the incident material instead of travelling on into the new material.
Refraction: light ray’s path is bent when it passes from one transparent material where its velocity changes.
- Factors affecting refraction: materials involved, angle of the incident ray of light, wavelength of incident ray.
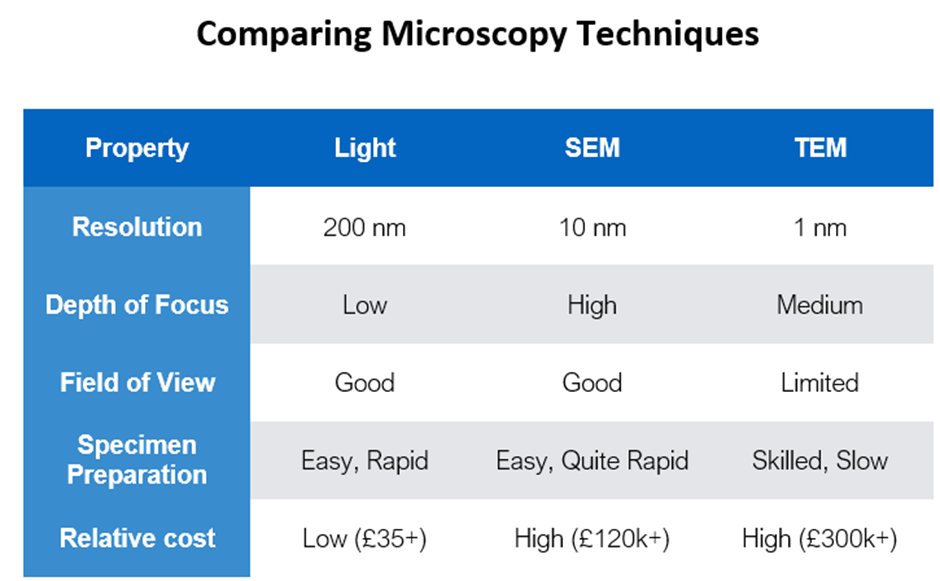
Resolution, depth of focus, field of view definitions + general principles
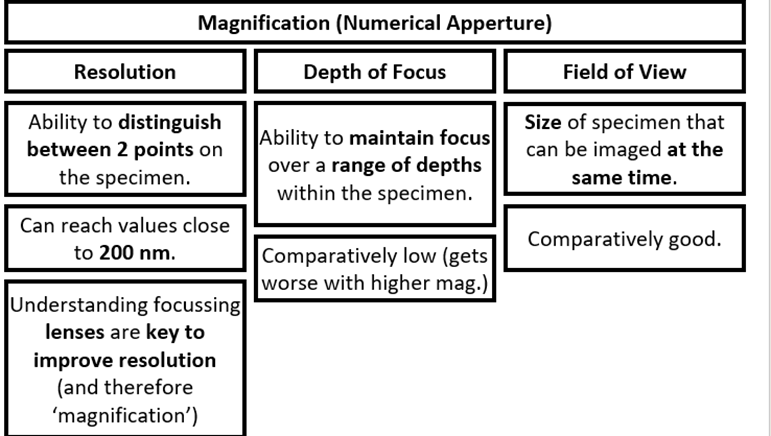
Types of Instrumentation: Microscopes + pros and cons (not darkfield and light field)
Stereoscopic microscope:
- Most frequently used in forensic science.
- 10-125x range.
- Resolution no close than 200 nm
- Large working distance.
- Good for bulky artefacts.
- Wide field of view, and great depth of focus.
- Great first step, when looking at physical features of trace evidence.
- Drawback: Has quite poor resolution.
Compound microscope:
- 40-50x range, up to 100x
- Transmitted and reflected illumination
- Precise focus and light intensity control
Comparison microscope:
- Allows point by point and side by side comparison to determine if two samples are from the same source.
- Two identical microscopes are connected to a single comparison eyepiece or screen.
- The viewer sees the image from both microscopes next to one another as an inset image to compare.
Fluorescence microscope:
- Similar to a stereoscopic or compound microscope, but the illuminating light is the ultraviolet wavelength range.
- Illumination causes some materials to fluoresce so they can be observed, counted and mapped.
- Fluorescent tagging can be used, but less common for trace evidence compared to biological sample or finger mark identification.
Polarised light microscopy:
- Normal light: waves vibrating in every direction perpendicular to the direction of travel.
- Linearly polarised light: waves vibrating in one direction.
- Normal light can become polarised if it passes through a material that only allows transmission of rays in a particular direction, such as a crystal, or film.
- Polarisation is useful in forensic microscopy when applied to anisotropic substances.
i.e., having a physical property which has a different value when measured in different directions.
Darkfield + brightfield microscopes
Entire field of view appears dark when there is no sample on the microscopic stage.
Sample placed on the stage appears bright against a dark background as only the scattered light is collected – contrast without staining.
Brightfield uses light from the lamp source under the microscope stage to illuminate the specimen.
To view a specimen, light rays that pass through it must be changed enough in order to contrast.
If a specimen has a refractive index similar to the surrounding medium, the image cannot be seen.
To visualise these materials, they must have a contrast with medium, or be stained, but staining can be destructive to specimens.
Equations to be aware of
Properties of waves related by formula: velocity (c) = frequency (f) x wavelength (lambda)
Both of the degree to which the light ray bends and the direction it bends are related to the refractive index values of the two substances.
The light ray angles and refractive indices (n) correspond to Snell’s Law.
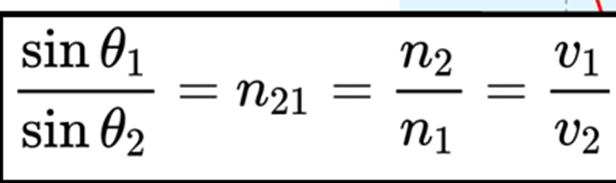
As light passes through a circular aperture, it is focused to a point. The spot size (d) is given by the diameter of the Airy disk, which is dependent of the light.
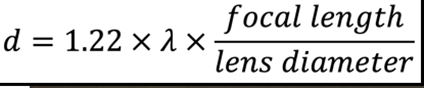
Focal length and lens diameter are parameters limiting resolution.
Becke Line + Single and Double variation methods
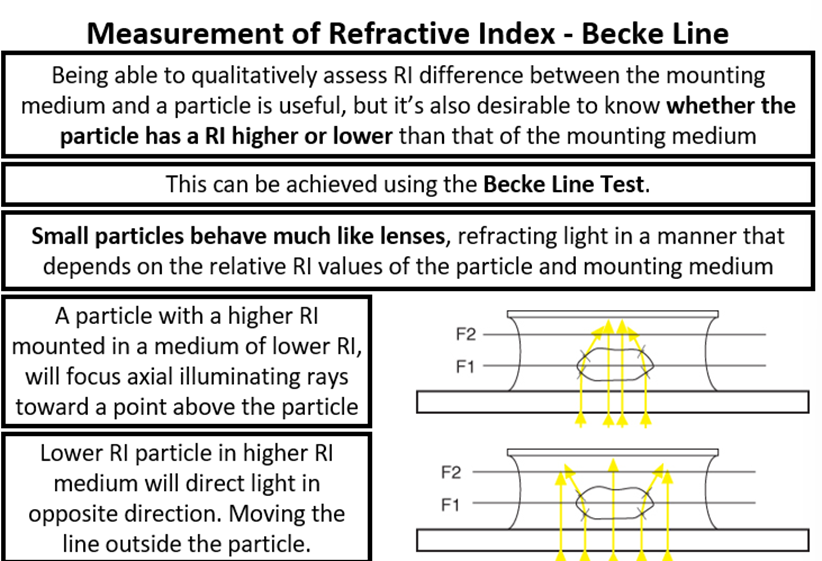
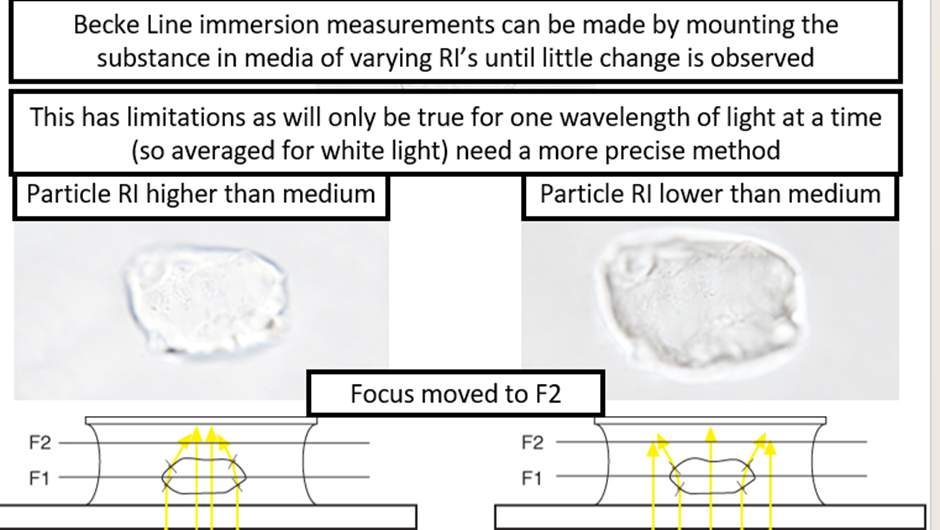
Single variation method is the more precise method we want:
1. Mount in a special high RI medium above that of the sample.
2. Fix light at a single wavelength (typically 589nm-sodium line)
3. Slowly heat the sample on a hot stage
4. The medium RI changes on heating much faster than the sample
5. The temperature of lowest contrast is noted
6. Compare to table of RI value corresponding to temperature
Double variation method is more precise – vary both the temperature and the wavelength in controlled manner.
Double variation methods:
Temperature is fixed and wavelength varied until a match is found
Temperature is then changed and process repeated to find new matching wavelength
Data plotted on Hartmann net – wavelength at the match temperature are plotted on the net and RI read off.
Equation of straight line established and converted to RI based on calibration data of the immersion liquid.
Comparing microscopy techniques - Light, SEM & TEM
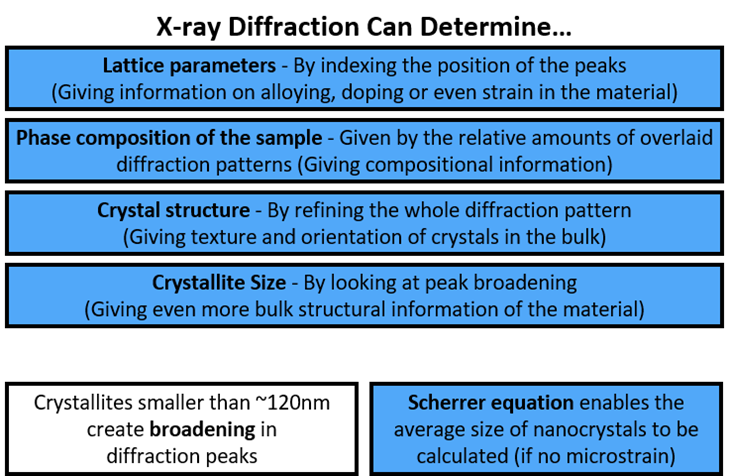
X-Ray diffraction definition + what it can determine
= used to establish the arrangement of atoms within a crystal structure and how they stack together
Determines:
Lattice parameters – by indexing the position of the peaks, giving information on alloying, doping, or even strain in the material.
Phase composition of the sample – given by the relative amounts of overlaid diffraction patterns
Crystal structure – by refining the whole diffraction pattern, giving texture and orientation of crystals in the bulk.
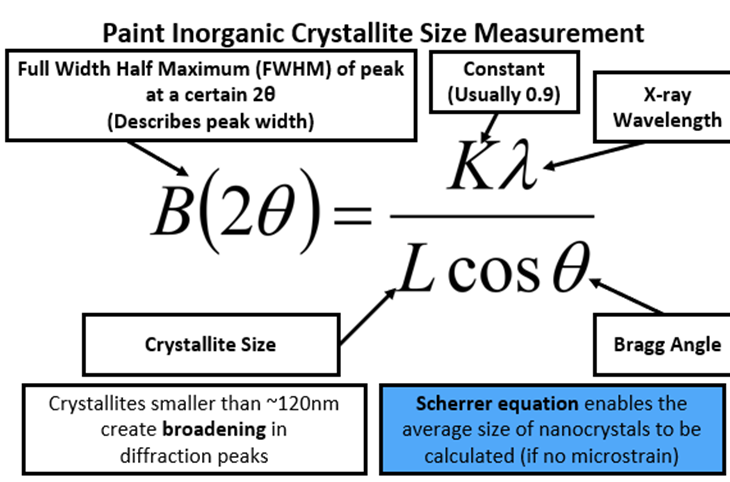
Crystallise size – by looking at peak broadening
Crystallites smaller than ~ 120nm create broadening in diffraction peaks
Scherrer equation – enables the average size of nanocrystals to be calculated.
Trace evidence pathway
Detect → Recognise → Recover → Examine → Interpret
Chemometrics, univariate, multivariate definitions
Chemometrics = “computationally intensive, multivariate statistical analysis applied to chemical systems or processes”.
Univariate = too simplistic, do not consider covariates (two aspects that influence each other).
Multivariate = data and trends are typically dependent on many variables.
What chemometrics does + aim of it
- Reduce complex datasets
- Identify and quantify sample groupings
- Optimise experimental parameters
- Isolate important variables and identify covariance
- Provide reproducible measures of data
- Allows for better visualisation of data
Aim = maximise output and quality of data analysis with minimal cost.
Applicability of chemometrics to forensic science
- Need for a ‘statistical framework'
- Replacement of ‘unique’ and ‘indistinguishable’ “match” with numerical uncertainties/probabilities in Q vs. K comparisons.
- Standard terminology across all disciplines.
- Counteract sources of bias.
- Much quicker than manual data interpretation à efficiency
- Models to predict trace evidence
- Does not negate the need for an expert
Smoothing, feature scaling, data transformations and scatter corrections
Smoothing = used to improve the signal to noise ratio for noisy data.
Feature scaling = considered as normalisation. Allows comparison of data sets, which may vary with different intensity profiles. Scale the peak information, so that the most intense peak has a maximum intensity of 1.
Data transformations = change the weighting of spectral information and are used when there is an imbalance in data information. Typically, these are logarithmic or square root transformations.
Scatter corrections = allows us to eliminate variation caused by effects unrelated to chemical nature: Standard Normal Variate (SNV) & Multiplicative Scatter Correction (MSC)
Supervised and Unsupervised chemometric methodologies
Supervised = pair learned variables such that known data can be used to generate new information about a sample, allows classification. (e.g., k Nearest Number, Linear Discriminant Analysis)
Unsupervised = aims to recognize patterns in data sets – no prior assumptions, does not allow classification. (e.g., cluster analysis, principal component analysis)
Exploratory Data Analysis (EDA) + scores plot info.
Allows us to start using our data to understand similarities and differences within a data set.
Scores Plot:
The scores plot is a map of the samples.
The position of those samples within the scores indicates whether it is a typical of the model or extreme.
The loadings plot is a map of the variables.
The magnitude of those variables (+/-) indicates the relative weighting assigned.
Interpretation of the scores in conjunction with the loadings allows understanding of why the samples cluster as they do, i.e. relates back to their chemistry.
Chemometric caveats
• Highly specific models.
• GIGO (garbage in, garbage out) – cannot compensate for bad data.
• Preprocessing is important but less is more.
• Sample size (admittedly difficult in trace analysis).
• Do the samples accurately reflect the population?
• Controlled sample collection?
• Reproducible/ repeatable results?
• Blind tests?
• Sample contamination or degradation.
• Appropriate analytical method?
• Correct parameters?
• Replicates?
• Outliers?
Human Element - Chemometrics
- Need to be aware of our own influence on how we treat and prepare our data for analysis, and we interpret the outputs.
o Beware of cognitive bias – chemometrics can only reduce subjectivity, not remove it.
o Analyst decides on data inputs, categories, number of PCs, etc. and data outputs.
o Never a substitute for human interpretation (years of experience/exposure to different cases)
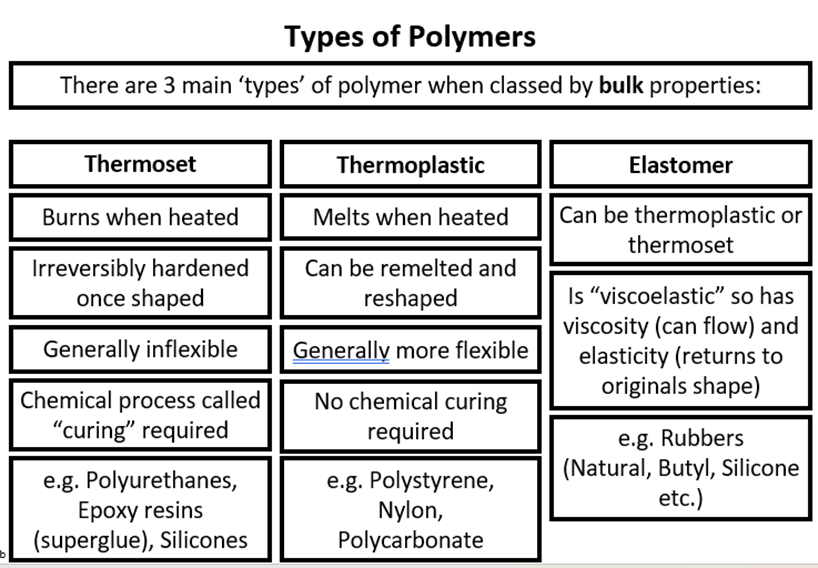
Three main types of polymer
High Density and Low Density Polyethylene (HDPE & LDPE)
HDPE:
- Containers and lids
- Food bottles, petrol tank
- Crates
- Pipes
- Higher density than LDPE
- Very low or no branching
- High crystalline content
- Less transparent than LDPE
- Stiffer and harder than LDPE
- Less gas permeable than LDPE
LDPE:
- Film and sheet packaging
- Toys
- Plastic bags
- Wire and cable coatings
- Lower density than HDPE
- Highly branched
- Low crystalline content
- More transparent than HDPE
- Forms good films
- More gas permeable than HDPE
Crystallinity definition + degree of crystallinity
= the regions of atomic ordering where intramolecular folding/stacking of adjacent chains occurs.
Affects a range of physical properties: Impact resistance, Young’s modulus, tensile strength, stiffness, crease, thermal behaviour, transparency
0 = completely amorphous
1 = completely crystalline
- Variation in refractive index with orientation, is a hallmark of amorphous materials, appearing isotropic.
- Crystalline polymers typically exhibit birefringence—they appear anisotropic under polarized light due to their ordered molecular structure.
Tensile strength
= how much something will stretch before it breaks (elongation stress)
- It increases with polymer chain length and crosslinking, more interaction between chains.
- Easier to stretch smaller things, as larger things require more matter to move, more force required.
- Account for sample length, and cross section.
Fibres definition + examples
= the basic unit of yarns and threads, which turn into fabrics, garments, textiles, etc.
Examples: clothing, bedding, carpets, seatbelts, bullet proof vests, toy stuffing.
Natural or manmade.
Natural fibres, “staple fibres”
- Plant → cotton, linen, seed, stem, leaf or fruit.
- Animal → silk, wool, cashmere, angora, camel.
- Mineral → asbestos only.
Four main types of natural fibres
Cotton:
- Is the most common fibre encountered in forensics. It is a cellulose-based polymer which is harvested from the seed pod to give ~2” long fibres which are raked to clean, pull and thin out. Fibres may then be dyed, before or after weaving.
Hemp:
- Derives from the cannabis sativa plant and is a cellulose based bast fibre.
- One of the fastest growing plants
- Uses: fabrics, building materials, paper and packaging.
Silk:
- Polymeric proteins formed by H bonded beta pleated sheets, so strong fibres.
- Silk fibres are produced industrially by silkworms, from cocoons.
- Silk is shimmery, due to prism structure.
Asbestos:
- Naturally occurring silicate mineral, forming thin fibrous crystals known as fibrils.
- Used as sound absorbing, fire resistance, and insulation, as is cheap and strong.
- Carcinogenic now (asbestosis) and is banned and removed from areas found.
Preparation of synthetic fibres
- Raw polymer converted into fibres via ‘spinning’ and extrusion through a spinneret device, creating multiple fibres.
- Spinneret introduces shape to the fibre.
- Fibres spun into bundles called filaments, can be used for their affordability, versality, and durability.
~ There are number of various spinning techniques and spinneret heads we can use which allow us to alter the characteristics of the fibre, e.g. shape, strength etc. As these are distinctive this makes exploring these characteristics and properties more forensically useful than chemical composition.
Recovery considerations - fibres
- Fibres may become dislodged quickly after deposition.
- Air dry wet clothing in controlled environment.
- Store in paper bags to prevent mould growth.
- Never package along with debris from the scene.
- Remember emergency personnel intervention.
Sample collection - fibres
- Preferable to submit entire item to lab.
- Druggist’s fold for small fibres, then label.
- Double package into evidence bag.
- Control sample – also packaged separately.
General Analytical Workflow
- Gross examination, recovery, and collection.
- Preliminary fit assessment
- All microscopic techniques.
- Micro spectrophotometry
- Infrared spectroscopy – identity
Raman spectroscopy - dyes and pigments
Single fibre analysis
Morphology
Cross section and diameter
Dichroism/pleochroism
Isotropy/anisotropy
Refractive index/birefringence
Fibre interpretations - considerations
- Class characteristics = traits common to a group
- Individual characteristics = traits that define and ID an item as different to others in the class.
- Number and location of fibres found
- Substrate considerations
- Multiple associations mitigate coincidental transfer
- Nature of contact – activity level propositions.
- New fabrics possess loosely adhering fibres.
- Old/damaged fibres may be damaged more.
- Tightly knit fibres shed less.
- Staple fibres shed more than filament fibres.
Fibre caveats
- Can never state fibre is ‘unique’
- Few databases for fibre origins
- Often overlooked as difficult to locate
- Expensive, time consuming, skilled analysis.
SEM for fibre analysis
- Reveals surface feature unseen in optical microscopy.
- Observes in-depth cross sections of fibres.
- High resolution imaging, easier to distinguish between fibres.
- Non-destructive – preserves the sample, allowing for further testing.
- Versality – handle a wide range of fibres, including natural, synthetic and composite materials.
Pleochroism + Retardation + Birefringence
Pleochroism = property in anisotropic materials causes it to show different absorption colours when it is exposed to polarised light coming from different directions.
Often the pleochroism of fibre found at a scene is compared to a fibre taken from a suspect, suggesting or disproving link between suspect and scene.
Retardation = velocities of light travel at different paces, one travels faster inside crystal. Slow ray is retarded and distance that the slow ray falls behind is called the retardation (R).
Birefringence = can calculate if we have the thickness and retardation of a material.
Paints, paint, coating + binders definitions
= coloured substance which is spread over a surface and dries to leave decorative or protective coating.
Paint = pigmented coating
Coating = wider term, covers any liquid which can be applied and dries to form a solid layer.
Binders = resins that help to bind the pigments together, provides adhesion to the surface which is being coated.
Dyes + pigments
- Collectively termed ‘colourants’ which are designed to enhance a product’s appeal and show colour.
- Dyes are soluble, pigments are insoluble particles dispersed in a matrix.
- Dyes are typically organic based; pigments are organic and inorganic components.
- Dyes uses are greater in textiles, pigments used in paints, inks, plastics, ceramics, cement, glass.
Colour defined by:
Hue - defines colour of shade, wavelength dependent.
Saturation, intensity, strength, chroma – purity of colour.
Brightness, luminance, value – lightness or darkness.
Tint = addition of white; shade = addition of black.
Inorganic pigments + uses + rutile + anatase
- Resistant to heat, light, weathering, solvents and chemicals, and cheaper.
- Pigments contribute to the ‘hiding power’ of the coating.
~ Titanium Dioxide is the most used white pigment due to excellent light scattering (high RI), heat and chemical stability, and low toxicity.
Rutile = more used, greater hiding power, slightly yellowish white,
Anatase = less used, less hiding power, bright white.
Uses:
- Sunscreens = UV blocker.
- Makeup = white pigment, UV blocker.
- Self-cleaning glass and solar cells.
- White pigments
- Cements, tiles and paints.
Metallic, Organic, and Extender pigments
Metallic pigments:
- Used in automative topcoats, and industrial finishes.
- Gives a deeper colour which changes when viewed from a different angle.
- Provides anticorrosion protection.
Organic pigments:
- Often called ‘lakes’ with bright colours being azo based.
- Used when a more bright or wider colour palate is used.
- Lower RI and so more transparency and less hiding power.
Extender pigments:
- Called fillers – do not add colour, may be used to provide higher hiding power, modify properties, reduce costs.
Additives - paints
Drying agents = speeds up polymerisation
Texturisers = smooths surface – solvents.
Emulsifiers = prevent separation, increases shelf life
Fungicides, biocides, insecticides.
Plasticisers – increases flexibility.
Paint evidence prepared for microscopy analysis
- Cross-section
- Thin peels
- Wedge-cut
- Stair-step exposure
Paint recovery considerations
- Often chips, flakes, fragments, delicate!
- Must not be fitted with J-Lar or acetate.
- Glass or plastic vial is preferable, then bag.
- Embedded flakes must not be removed at scene.
- Paint transfers should not be lifted – submit item.
- Do not refrigerate or freeze evidence.
Paint interpretation - analysis
• Number of fragments and their physical dimensions
• Number, sequence, thickness and colour of layers
• Application method → brush strokes? Air bubbles?
• Surface topography → wrinkle finish, hammered finish
• Size and distribution of pigments (speciality?)
• Defects, weathering, delamination, corrosion
• Data base queries Car resprays/wall repainting → increase distinctiveness
• Cross-transfer → increased significance and value
• Limited research on spray paint variability and transferred paint persistence
X-Ray Fluorescence (XRF) Theory
- Absorption of radiation at one energy and re-emission at a different energy.
- When excited by an external X-Ray, an electron from inner shell is ejected from the atom, creating a vacancy.
- An electron from the outer shell fills the vacancy, which leads to…
Energy of particle being lowered.
Leads to emission of this difference in energy in the form of an X-Ray which is unique to element.
In turn, produces a vacancy in the outer shell, which then needs to be filled releasing another X-Ray etc.
X-Ray Diffraction determines:
Cosmetics + decorative cosmetics definitions
= a product applied to the external parts of the body to enhance its appearance, for example, cleaning, perfuming, protecting and keeping it in good condition.
Decorative cosmetics = any makeup examples, i.e., foundation, bronzer, blusher, etc.
Typical cosmetic ingredients
Typical cosmetic ingredients:
- Emulsifiers = help components mix
- Consistency regulators = controls consistency
- Moisturisers
- Dyes/pigments = provides colour/shade
- Active ingredients = antioxidants, humectants, acids, antiseptics
- Fragrances
- Preservatives = improves product shelf life
Special effect pigments
- Pearlescent → subtle colour and bright white reflection, less intense effect than metallic
- Interference (iridescent) → light reflection and refraction, colour changes relative to angle of observation/illumination, ‘rainbow-like’, CD surface etc.
Modern cosmetic trace value
Cosmetic use is independent of age, gender, ethnicity, socioeconomics.
It is transferred, particularly with violent force, and is sometimes difficult to wash out
Cosmetic variety and application
Cosmetics useful as trace evidence, due to a huge variety of products, manufacturers, shades, tones, etc., leads to complex and unique mixtures making it beneficial for trace evidence.
Cosmetic probative value
Even though cosmetics are not fully explored well, they can have extensive probative value:
- Cases with lack of physical evidence
- Uncommon cosmetics or layering combinations increase value
- Makeup leaves deposits – enables for an event to be reconstructed.
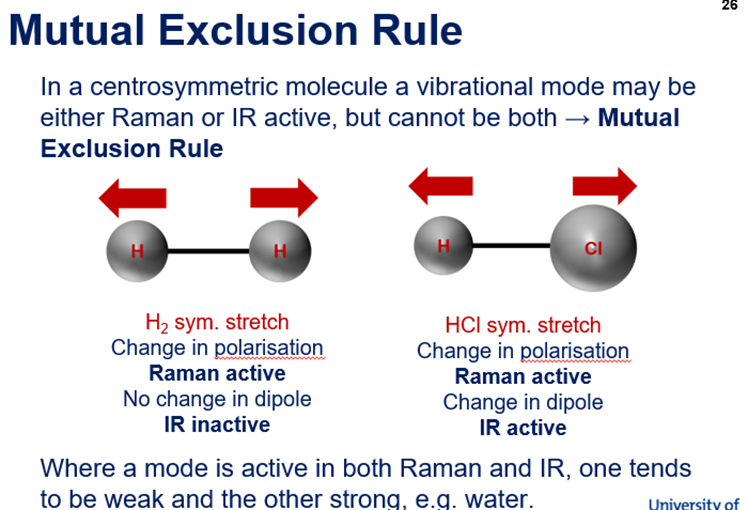
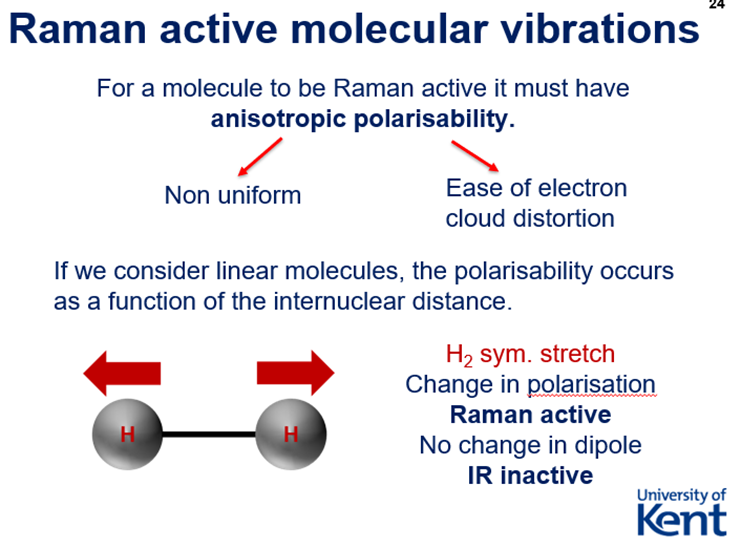
Raman Scattering - Rayleigh, Stokes, Anti-Stokes + Rules
Recovery Considerations - cosmetics
- Air dry wet garments in controlled environment.
- Store in paper bags to prevent mould growth.
- Cosmetics adhere to garments/bedding/carpets better than fibres, making it easier to collect evidence.
Components in cosmetics
Carrier vehicle – water and siloxanes (oils and waxes)
Titanium oxide or zinc oxide – coverage for UV protection
Talc or kaolin – absorbents
Isododecane, dimethicone – emollients
PEG, polymers – emulsifiers
Pigments and effects
Borosilicate glasses – effects or bulking agent
Cosmetics - optical microscopy
Colour matrix/particles
Distribution of pigments
Particle morphology
Surface topology
Mica vs. synthetic (also SEM-EDX)
Borosilicate glass
PMMA/silica spheres (also RI measurement)
Component encapsulation
Considerations for microscopic cosmetic trace analysis
- Consider oblique or alternate lighting – particles reflect differently, e.g., interference pigments
- alternate between black, grey and white backgrounds to facilitate colour determinations.
- Q vs K comparisons must be performed side-by-side by using the same background colour.
- Transmitted light for observing pigment distribution, reflected light for layers or textures - use both.
Spectroscopic analysis - cosmetics
- SEM provides a way to look at particle size and morphology.
- EDX provides elemental info, linked to specific areas or particles.
- XRF provides wider elemental info linked to a sample.
- X-Ray diffraction provides info about crystalline products, info about inorganic pigments, clays, organics.
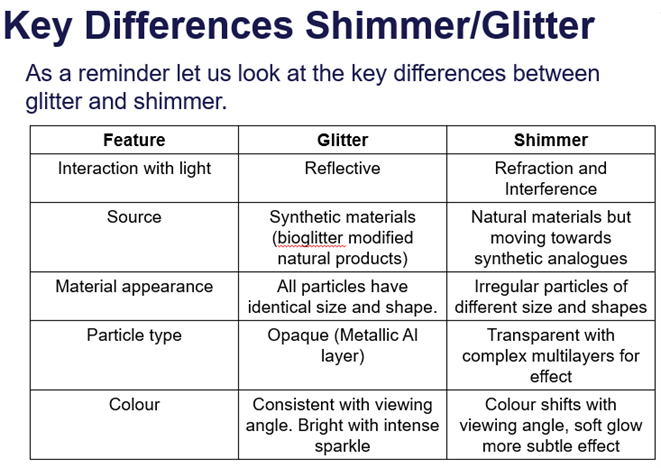
Glitter, shimmer, pearlescence, iridescence definitions
Glitter = multilayer synthetic flat particles. Plastic coated in reflective aluminium layer and transparent colour layer.
- Visual effects of glitter are observed as a result of reflection of light from aluminium surface, acts like a mirror.
- Glitter is considered a microplastic, bio glitter is produced as an alternative, without the microplastics.
Shimmer = particles scatter light. Colour softer than glitter, provides iridescent and pearlescent effects.
Pearlescence = materials which reflect white light to give pearl like effects, through use of interference particles comprised of a Mica substrate, coated with titanium oxide.
Iridescence = same as pearlescence, but name given for shimmer particles which provide the addition of colour. Additional layer added to titanium oxide to provide colour.
Key differences between Glitter and Shimmer
Recovery considerations + forensic value - glitter and shimmer
Recovery considerations:
- Treat similarly to paint evidence.
- Use a high light intensity source.
- Must NOT be lifted with J-Lar or acetate – post its.
- Glass or plastic vial is best option, then bag.
- Embedded particles must not be removed at scene.
- Do not refrigerate or freeze.
Forensic value:
- Easily transferable and persistent.
- Nearly invisible, until catches light.
- Quickly collected, separated, concentrated.
- Easily characterised, only single particle required.
- Resistant to degradation
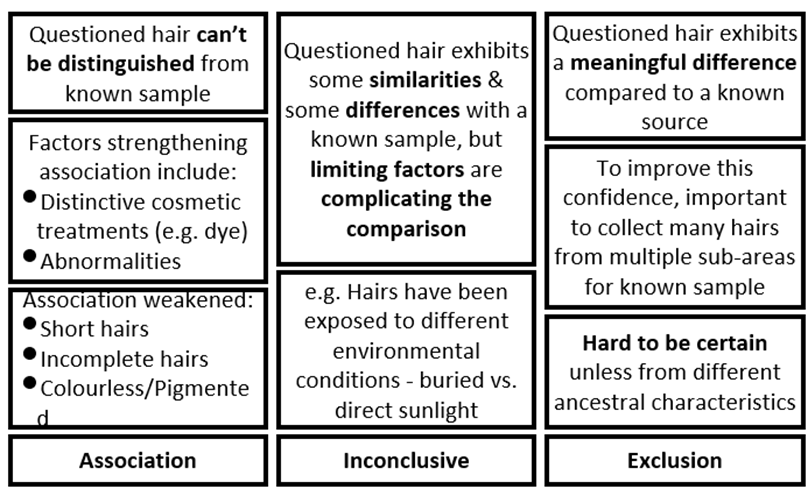
Hairs - evidential value for trace evidence
- Found on all humans and other mammals
- Easily transferred from one person/object to another
- Constantly being produced and shed in environments.
- Highly stable, resisting physical and chemical degradation.
- Hairs can be distinguished from each other, and with DNA testing.
- Provides investigative leads and reconstruction of events.
Types of hairs
Lanugo = hairs formed in utero, fine and unpigmented hairs. Shed before or shortly after birth.
Vellus = fine, short, unpigmented or lightly coloured hairs present on almost all skin surfaces, apart from palms of hands and soles of feet.
Terminal = typical hairs visible oh children and adults.
- Primary hairs: head, eyelash, eyebrow.
- Secondary hairs: pubic, underarm, beard.
~ Forensic analysis of hairs restricted to terminal.
Histology of hairs
Cuticle = outermost layer of hair, responsible for chemical resistance.
Cortex = main bulk of hair, responsible for mechanical properties of hair, contains most of pigment granules of hair, giving hair colour.
Medulla = innermost layer of hair shaft, not present in all hairs.
Cell membrane complex = binds all cells together.
Follicle = where hair grows from and changes size and shape throughout hair cycle.
Physiology of hairs - hair cycle
Anagen = active growing phase of hair, from follicle root outwards from skin.
Catagen = transition phase when growth slows and eventually stops.
Telogen = resting phase when minimal force is required to remove hair and natural shedding (exigent) likely to occur.
Estimated that 100,000-200,000 hairs on average scalp.
Around 30-100 hairs shed each day.
Around 1 cm of hair growth per month.
Hair does not turn grey, pigment stops being produced, giving appearance of grey/white.
Collection + isolation of hair evidence
Forceps – better for individual hairs, careful not too much pressure.
Tape lift – collecting hairs from large surfaces – combings also possible.
~ Should be collected using combination of plucking and combing.
Features evaluated in microscopy - hair evidence
Colour
Cosmetic treatments – bleached/dyed
Thickness range
Cross-sectional shape – round, oval, flattening etc.
Shaft irregularities – buckling, twisting.
General damage – split, frayed, broken, burned.
Biological damage – insect bites, fungal, bacterial activity.
Adhering material – blood, nits, residues.
Non-root morphologies – rounded, cut, broken.
Types of ionisation techniques used in MS
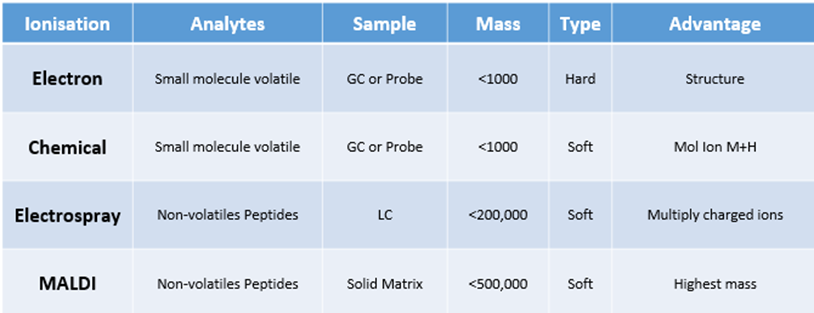
MALDI = Matrix-Assisted Laser Desorption Ionization
Types of mass analysers used in MS
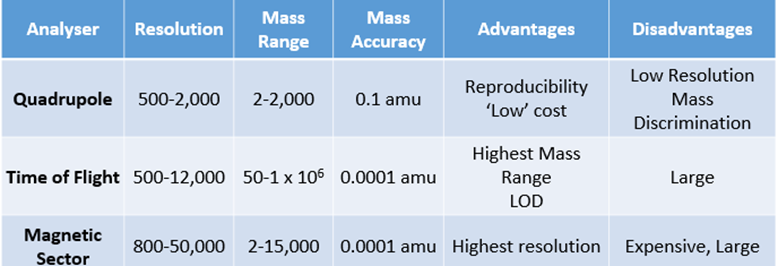
3 main conclusions drawn from interpretation of microscopical hair analysis
Glass composition
Soda-lime glass most common:
- Silicon dioxide
- Calcium oxide
- Sodium oxide
Other components:
- Boron oxide added to improve heat durability in cookware, glassware, and automobile headlamps.
- Silver added in sunglasses, and strontium in TV screens to absorb radiation.
Glass breakage + transfer
- Determination of side impact by comparison of hackle marks or rib marks.
- Percussive cone more likely for projectiles impacting glass.
- Transfer from a crime scene most likely in hit-and-run and ram-raids.
- Produce large shards but is usually only small fragments that transfer on clothing.
Physical examination of glass + comparing it to potential quartz and minerals
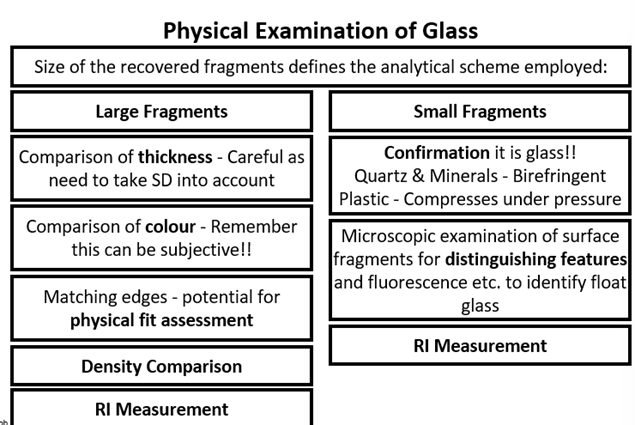
Glass Analysis - SEM-EDX + uXRF
- Minimally destructive, can analyse tiny fragments, sample preparation is relatively easy.
- Detection limit of 1000 ppm, so limited in sensitivity to major elements in glass – most discriminating trace is left undetected in glass.
- Poor precision, due to variation in fragment orientation, shape, thickness, affecting measurements and quantitative analysis challenging.
- SEM-EDX good, but micro XRF (uXRF) preferred for glass.
uXRF:
- Uses same detection as SEM-EDX but excites using an X-ray source rather than beam of electrons, penetrates much deeper into glass.
- Bulk analysis technique, less affected by fragment shape with a detection limit improved to 10-50 ppm.
- Measure small fragments.
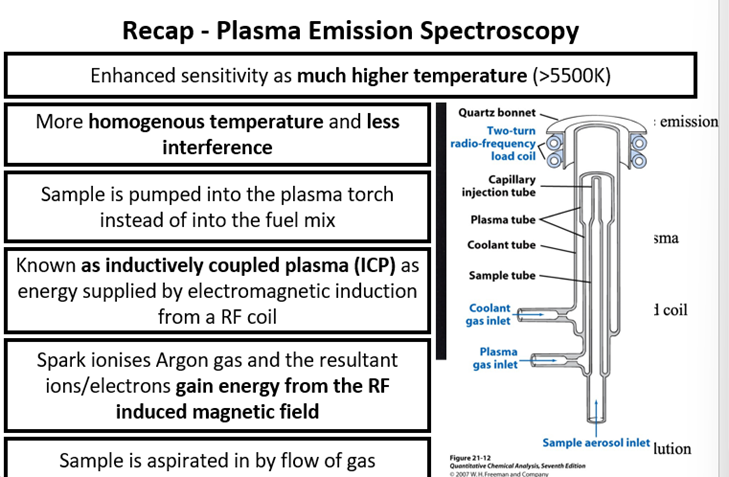
Glass Analysis - Plasma Emission Spectroscopy
Diatoms + value for forensics
- Type of phytoplankton, eukaryotic algae
- Making up a lot of the Earth’s biomass
- Nearly half of the organic material found in ocean
- Contribute significantly to sequestering carbon and producing oxygen
- Vital for part of oceanic food chain, and producing oxygen
- Consumed in foodstuff
- Inhaled in low quality cigars
- Contamination of equipment
- Diverse group of species, small size range so easily enters inside body organs
- Hard silica cell wall is resistant to chemical changes, helps in recovering of intact structure of diatoms from body or clothes.
- Diatoms often retrieved when no other evidence types can be
Foraminifera + phytoliths as trace evidence
Single celled organisms – live on sea floor and have external ‘shell’.
Limited usage to date as trace evidence
Sediments can be sourced in building and industry – this increases their forensic trace evidence value.
Plant microfossils made of silica found in some plant tissue, persisting after death and decay of plants.
Released into soil or sediment after plant death – great forensic value.
Composed of silicon dioxide, provides a RI.
Used alongside soil and environmental trace evidence, hard solely on its own
Pollen as trace evidence
Powder containing male genotypes of seed-producing plants – dispersed in order to reproduce by wind, water or animals.
Surface textures can be diverse and identifiable to a particular plant
Pollen persists for a long time
Lack of skilled people to deal with pollen as trace evidence, and limited national or international databases of pollen
- SEM considered a standard for pollen evidence but is time consuming.
- Semi-automated TLM and molecular barcoding used as an alternative.
Geoforensic Trace
= variety of sizes, not limited to soil/rocks, also includes pollen, plant material.
- Rocks generally static and stable
- Soils generally dynamic and unstable
- Sediments, soils, dust, rocks as mixtures of organic/inorganic particles.
Nomenclature: rock, soil, sand, silt, clay, loam, sediment, mud, dirt
Rock = naturally occurring solid mass at surface of Earth’s crust.
Soil = living environment made up of minerals, organics, inorganics, water and plant matter
- Sand, silt, clay, loam à soil constituents defined by size.
Sediment = loose material which has been transported away from its original location by natural processes.
- Mud = type of sediment, liquid or semi-liquid, typically comprised of fine-grained loam, silt, and clay.
Dirt = non scientific term. Often used to explain microdeposits.
Types of rocks
Classified on how they are formed:
Igneous = from volcanic activity (cooled lava)
Sedimentary = compacted sediments
Metamorphic = rocks which have changed under temperature and pressure.
Soil - components
Pore space = water/air frictions, allowing water/air to circulate through the soil.
Organic matter = made up of Humus (decayed animal and plant life), roots and organisms. Humus is the source of plant nutrients, increases the soil’s ability to retain water.
Minerals = mineral matter is produced by weathering of the crust, and rocks materials.
Soil - formation
- Properties of soil are dependent on how soils are formed, dependent on interlinked factors:
Parent material, age, climate, plant animals, slope.
Environmental Trace - climate

Soil texture vs Loam

Anthropogenic Soils

Environmental Trace - physical characteristics measured
- Weight and volume
- Colour and texture (sieve test)
- For individual particles – dimensions, surface area, perimeter, shape (form, sphericity, regularity).
Environmental Trace - colour + pH
Colour determination:
- Using Munsell’s soil charts, a 3D model breaking colour down into a numerical representation given as H, V/C (hues, values, chroma).
- Measurement of pH of soil, acidic/alkaline, and measuring the electrical conductivity of soil. Converts the electrical conductivity to total dissolved solids, provides a measure of the nutrients in a solid.
Environmental Trace - IR, Raman, SEM-EDX, XRD, XRF
- FTIR measurements allow for an understanding of the clay minerals and hydroxides and oxyhydrides.
- Raman can provide vibrational information about oxides and silicate minerals.
- SEM-EDX provides details of the microstructure of components of the soil, EDX providing elemental information.
- XRD can be used for exploring crystalline mineral contents in a soil.
- XRF provides elemental information, elements with mass > Na.