Lecture 15 - Lipids and Membranes
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
72 Terms
What defines a lipid chemically?
Lipids are insoluble in water but soluble in non-polar solvents.
They are a diverse group of molecules that share this property, rather than a common structure.
What are the major biological roles of lipids?
Components of membranes*
Storage form of carbon and energy*
Insulation against thermal, electrical, and physical shock
Protective coatings (e.g., waxes) to prevent infection or water loss
Precursors to other substances
Some vitamins and hormones
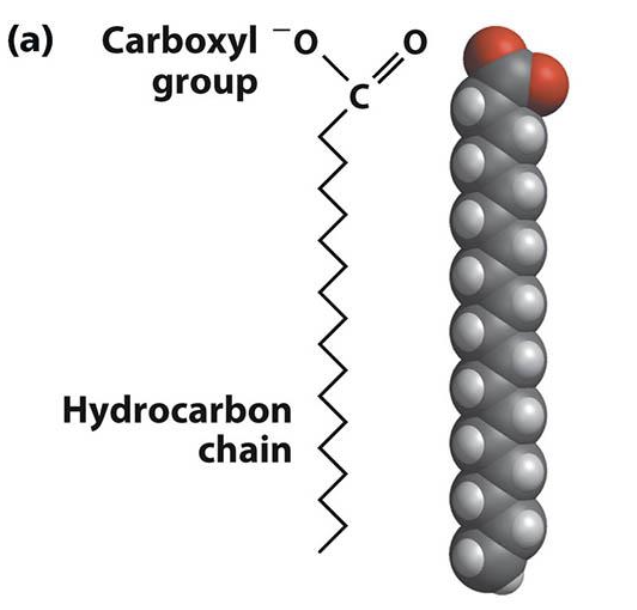
What are fatty acids (FAs)?
Fatty acids are amphipathic long-chain aliphatic carboxylic acids.
They have a hydrophilic carboxyl group and a hydrophobic hydrocarbon chain.
Are fatty acids typically found in free form in cells?
No — fatty acids are rarely found free in cells.
They are usually part of complex lipids like triglycerides or phospholipids.
How are fatty acids released from fats?
They are released by hydrolysis of fats (e.g. triglycerides).
Which type of fatty acids are least soluble in water?
Long-chain saturated fatty acids are the least soluble in water due to their extensive non-polar hydrocarbon tails.
Why are long-chain fatty acids less soluble in water?
Because longer hydrocarbon chains are more hydrophobic, they cause more structured (caged) water around them, making them less soluble in water.
Why are longer hydrocarbon chains more hydrophobic, and what does this mean for water solubility?
Longer hydrocarbon chains have more non-polar C–H bonds, increasing hydrophobicity. In water, these chains can't form hydrogen bonds, so water molecules form ordered "cages" around them. This caging is energetically unfavorable, reducing solubility.
What structural features are most common among naturally occurring fatty acids?
Most are linear, unbranched monocarboxylic acids with an even number of carbon atoms (typically C12–C24). Variants like shorter, longer, odd-numbered, branched, or cyclic chains are much less common.
What is a common structural pattern in unsaturated fatty acids with multiple double bonds?
When multiple double bonds are present, they are almost always separated by a single methylene group (–CH=CH–CH₂–CH=CH–). C18 and C20 unsaturated fatty acids are especially common.
In unsaturated fatty acids, double bonds are usually separated by a methylene bridge:-CH=CH–CH2–CH=CH-
What is the typical configuration of double bonds in naturally occurring unsaturated fatty acids, and why are trans fatty acids important?
Naturally occurring unsaturated fatty acids almost always have cis double bonds. Trans fatty acids, often produced by industrial hydrogenation, are linked to increased risk of heart disease.
saturated fatty acid structure
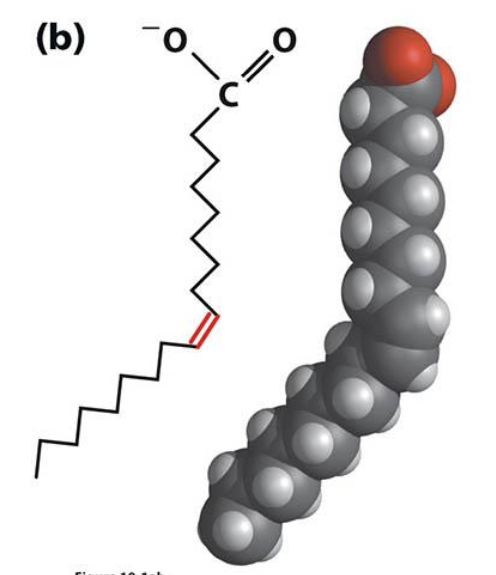
monounsaturated fatty acid structure (when a double bond is introduced it causes a bend in the molecule)
What is lauric acid?
Lauric acid is a saturated fatty acid with 12 carbon atoms (C12:0).
What is palmitic acid and its significance?
Palmitic acid is a saturated fatty acid with 16 carbon atoms (C16:0). It makes up about 26% of human fat.
What is stearic acid and its significance?
Stearic acid is a saturated fatty acid with 18 carbon atoms (C18:0). It makes up about 5% of human fat.
In fatty acid notation (e.g., C18:0), what do the numbers mean?
The first number is the total number of carbon atoms (COOH carbon is #1), and the second number after the colon is the number of double bonds. For saturated fatty acids, this is 0 (e.g., C18:0 means 18 carbons and no double bonds).
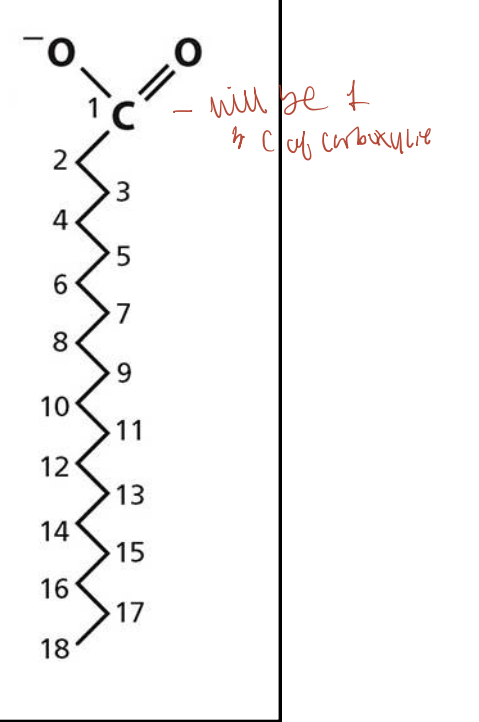
What does the ∆ (delta) notation in fatty acids indicate?
It shows the position of the double bond(s) counting from the carboxyl carbon (#1). For example, ∆9 means a double bond between carbon 9 and 10.
How are multiple double bonds typically arranged in naturally occurring unsaturated fatty acids?
They are almost always separated by a single methylene group (–CH₂–), creating a –CH=CH–CH₂–CH=CH– pattern. This means the double bonds occur every third carbon, making them non-conjugated.
What is palmitoleic acid?
Palmitoleic acid is an unsaturated fatty acid with 16 carbons and one cis double bond at ∆9 (between C9 and C10). Notation: ∆9C16:1.
Describe oleic acid.
Oleic acid is an 18-carbon unsaturated fatty acid with one cis double bond at ∆9. Notation: ∆9C18:1. It makes up about 46% of human fat.
Why are the double bonds in linoleic, α-linolenic, and γ-linolenic acids said to be "3 apart"?
Because their double bonds are separated by a single methylene group (–CH₂–), forming a –CH=CH–CH₂–CH=CH– pattern. This results in double bonds occurring every three carbon atoms.
Linoleic acid: Δ⁹,¹²
α-Linolenic acid: Δ⁹,¹²,¹⁵
γ-Linolenic acid: Δ⁶,⁹,¹²
What does it mean when a fatty acid is called an omega-6 (ω-6) fatty acid?
In omega (ω or n) nomenclature, the position of the last double bond is counted from the methyl (CH₃) end of the fatty acid.
For example:
Linoleic acid (18:2n-6)
γ-Linolenic acid (18:3n-6)
Both are omega-6 fatty acids because their last double bond is six carbons from the methyl end.
What is the typical pKa range of fatty acids and what is their charge at physiological pH (~7)?
pKa is about 4.5–5.0; at pH 7.0, the carboxyl group is deprotonated (negatively charged).
How does fatty acid chain length affect melting point?
Melting point increases with increasing chain length.
How does the number of double bonds in fatty acids affect their melting point?
Melting point decreases as the number of double bonds increases.
Up to what carbon chain length are fatty acids water-soluble?
Fatty acids are water-soluble up to C6; longer chains are soluble only in non-polar solvents.
What does amphipathic mean in the context of fatty acids?
Fatty acids have both hydrophilic (carboxyl group) and hydrophobic (hydrocarbon chain) regions.
How do fatty acids contribute structurally in water?
They form micelles.
Why are these fatty acid properties important biologically?
They influence membrane fluidity or stiffness depending on fatty acid composition.
What are the key properties of fatty acids?
pKa around 4.5–5.0; mostly deprotonated at pH 7.0
Melting point increases with chain length, decreases with more double bonds
Water-soluble only up to ~C6; longer chains soluble in non-polar solvents
Amphipathic (hydrophilic carboxyl + hydrophobic chain)
Form micelles in water due to amphipathic nature
Affect membrane fluidity and stiffness depending on composition
Why do fatty acids form micelles in an aqueous environment?
Because fatty acids are amphipathic, their hydrophobic tails cluster together away from water while their hydrophilic heads interact with water, forming micelles. This arrangement is thermodynamically favourable as it minimizes the system’s free energy.
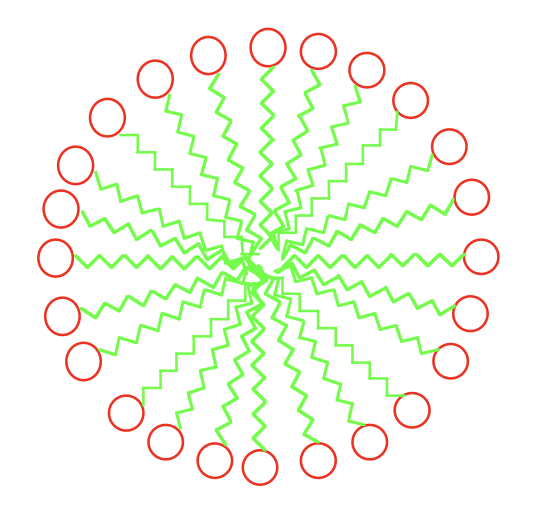
What are the two main types of lipids?
Storage lipids (neutral)
Membrane lipids (polar)
What are the main classes of membrane lipids?
A) Glycerophospholipids
B) Sphingolipids
i) Sphingomyelins
ii) Glycosphingolipids
a) Cerebrosides
b) Globosides
iii) Gangliosides
What are storage lipids typically called?
Triacylglycerols or triglycerides.
What are triacylglycerols made of?
Glycerol backbone esterified with three fatty acids.
What types of acylglycerols can form?
Mono-, di-, and triacylglycerols, depending on how many fatty acids are attached.
Which form of acylglycerols is most common?
Triacylglycerols, with three fatty acyl chains.
Can all three fatty acids in a triacylglycerol be the same?
Yes, for example in tristearoylglycerol (tristearin), all are stearic acid (C18).
acylglycerol form
What is the function of intestinal lipases?
They catalyze the enzymatic hydrolysis of dietary triacylglycerols (TAGs) into fatty acids and glycerol for absorption into the body.
What do lysosomal lipases do?
They cleave ester bonds between glycerol and fatty acids in lipids stored inside cells.
What is the role of endothelial lipases?
They help release fatty acids from lipoproteins for uptake into cells.
How are some lipases involved in signaling?
They cleave membrane lipids to produce signaling molecules.
What is the effect of phospholipases in venom?
They break down plasma membranes, compromising cell integrity.
How does the melting point of triacylglycerols vary?
It increases with fatty acid chain length and decreases with increasing unsaturation.
What is the most abundant family of lipids?
Triacylglycerols (triglycerides), which include fats (less unsaturated) and oils (more unsaturated).
Why are triacylglycerols efficient for energy storage?
Their carbon atoms are more reduced than in carbohydrates, providing ~2x more energy per unit mass.
What role do triacylglycerols play in insulation?
They are stored under the skin (e.g., in seals, walruses, and humans) to prevent heat loss.
How do triacylglycerols aid in buoyancy?
In sperm whales, changes in the density of spermaceti oil with temperature allow buoyancy control at different depths.
What are the properties of triacylglycerols (TAGs)?
Melting point varies similarly to single fatty acids— increases with chain length and decreases with unsaturation.
TAGs are the most abundant family of lipids: fats (less unsaturated) and oils (more unsaturated).
Serve as energy storage in fat cells (adipocytes) and oil seeds; fat has ~2x energy per unit mass compared to carbohydrates due to more reduced carbon.
Provide insulation, stored under skin in seals, walruses, penguins, and humans.
Aid buoyancy, e.g., sperm whales adjust buoyancy as spermaceti oil density changes with temperature.
How do the fatty acid compositions of butter, olive oil, and beef fat relate to their physical state at room temperature
Butter (soft solid):
~Equal amounts of C16 & C18 saturated and unsaturated FAs; some C4–C14 saturated.Olive oil (liquid):
High in C16 & C18 unsaturated FAs; low in saturated FAs; very little C4–C14.Beef fat (hard solid):
High in C16 & C18 saturated FAs; some unsaturated; very little C4–C14.
Explanation:
The more saturated the fat, the higher the melting point — hence, beef fat is solid, butter is softer, and olive oil is liquid at room temperature.
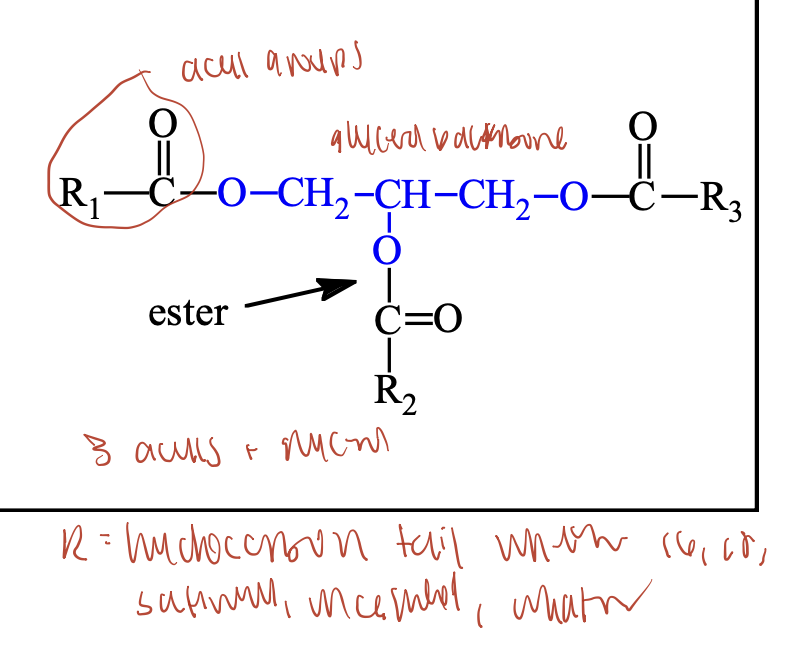
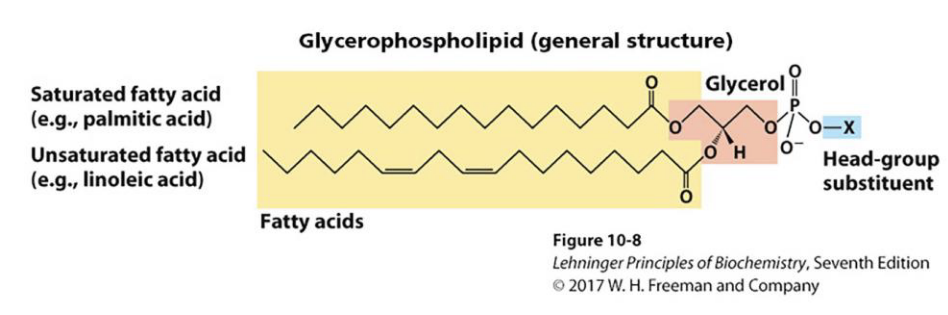
What are glycerophospholipids?
Glycerophospholipids are polar membrane lipids derived from phosphatidic acid, which consists of a glycerol backbone with:
Two fatty acids (at positions 1 & 2)
A phosphate group (at position 3)
Often, the phosphate is linked to another polar head group.
Structure:
CH₂–O–C(=O)–R₁
CH–O–C(=O)–R₂
CH₂–O–P(=O)(O⁻)–X (polar head group)
- head group gives identity
What are the most common head groups in glycerophospholipids?
Ethanolamine → Phosphatidylethanolamine (looks like R group of lysine)
Choline → Phosphatidylcholine (lecithin)
Serine → Phosphatidylserine (looks like serine)
What is the charge of phosphatidylethanolamine and phosphatidylcholine?
Both are zwitterionic at physiological pH (carry both +1 and −1 charges).
What is the net charge of phosphatidylserine at physiological pH?
−1, due to +1 on the amine and −2 from phosphate and carboxyl groups.
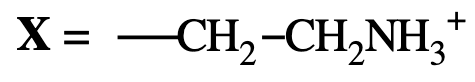
phosphatiylethanolamine
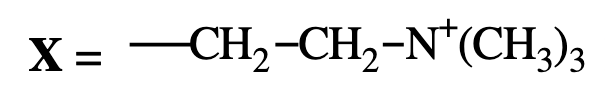
phosphatiylcholine
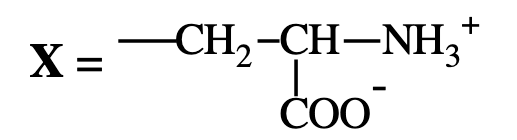
phosphatiylserine
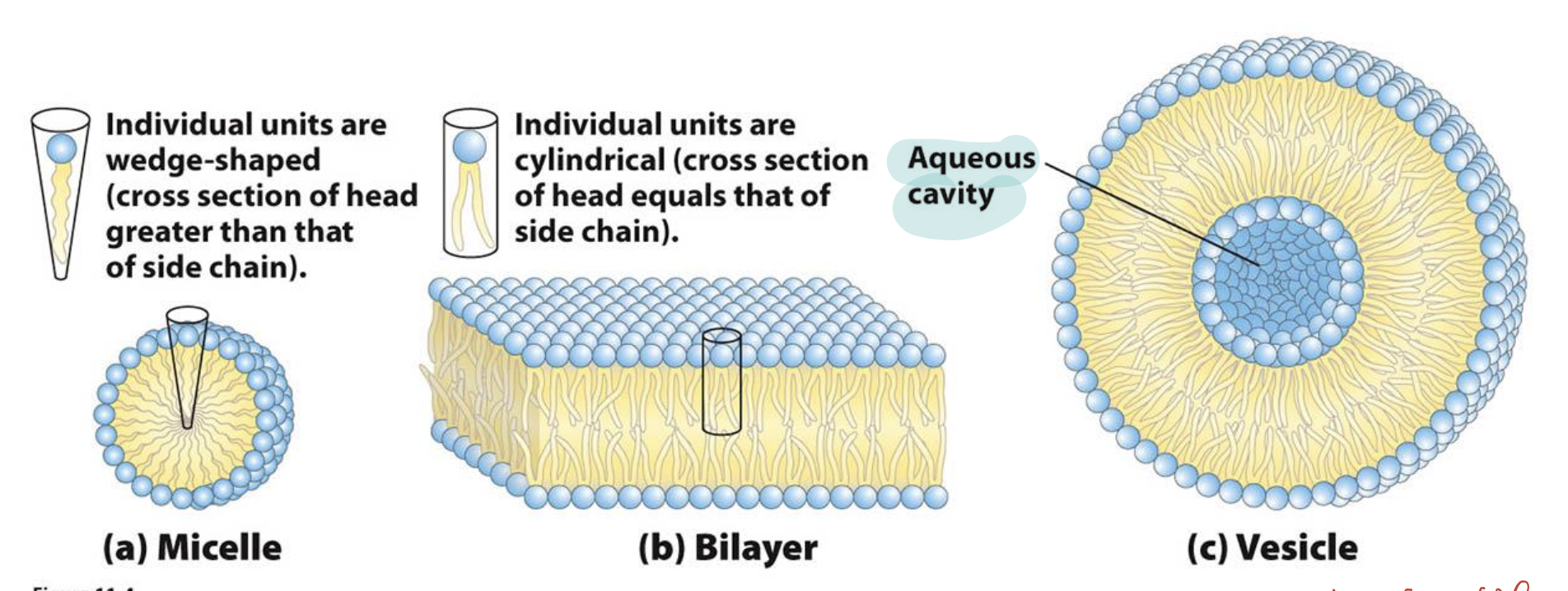
Why do phospholipids form bilayers in aqueous environments?
Phospholipids are amphipathic, with a polar head and non-polar tails. Due to the hydrophobic effect, they self-assemble into bilayers, where the hydrophobic tails face inward and polar heads face water, forming the basis of biological membranes.
Do phospholipids form micelles or bilayers, and why?
Phospholipids generally form bilayers, not micelles, because they have two bulky hydrophobic tails. This shape prevents the tight packing needed for micelle formation and favors bilayer structures instead.
What structures can curved phospholipid bilayers form?
Curved phospholipid bilayers can form vesicles and liposomes, which are closed spherical structures enclosing an aqueous compartment.
What is a lysophospholipid?
A lysophospholipid is a phospholipid with one fatty acyl chain removed, typically by the action of specific phospholipases like A1 or A2.
What enzymes cut phospholipids?
Specific phospholipases—A1, A2, C, and D—each cleave different parts of phospholipids.
What are sphingolipids and where are they found?
Sphingolipids are membrane lipids derived from sphingosine (not glycerol) and are especially important in the brain and peripheral nervous system. They are not glycerol derivatives — they are derived from sphingosine, a molecule with a long hydrocarbon chain.
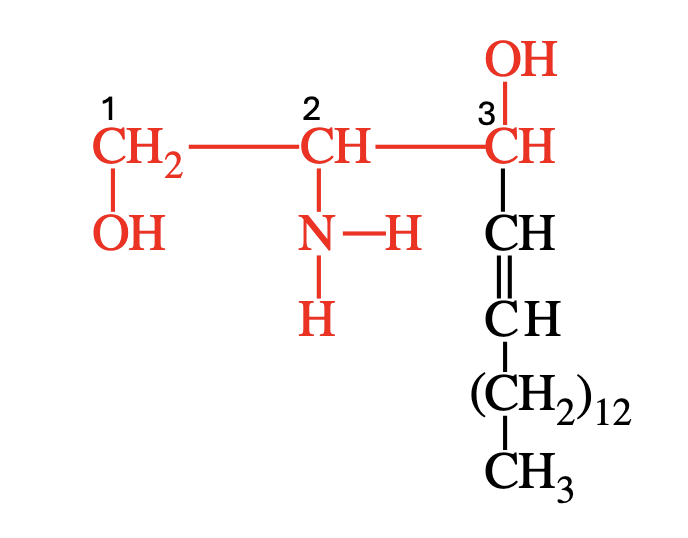
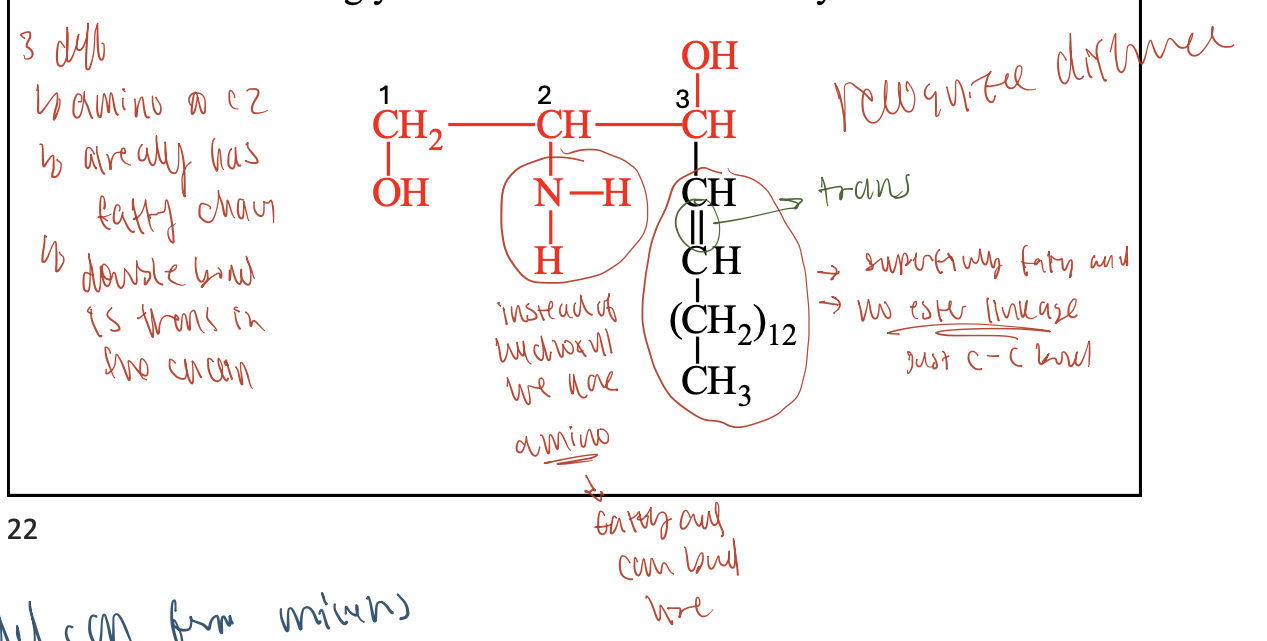
How does sphingosine differ from glycerol?
Backbone: Sphingosine has 2 hydroxyl groups and 1 amino group (–NH₂), while glycerol has 3 hydroxyl groups.
Built-in hydrocarbon chain: Sphingosine includes a long nonpolar chain; glycerol does not.
Fatty acid attachment: Sphingolipids use an amide bond (via –NH₂); glycerolipids use ester bonds (via –OH).
What is a ceramide, and what are the main subclasses of sphingolipids?
A ceramide is formed when a fatty acid is attached to sphingosine via an amide linkage. It is the structural base of all sphingolipids. The three main subclasses are:
i. Sphingomyelins
ii. Glycosphingolipids
iii. Gangliosides
(The subclasses differ in their polar head group, X.)
glycerophospholipid
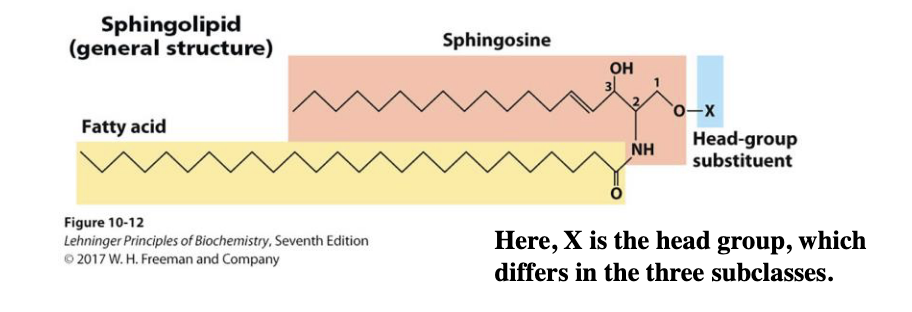
sphingolipid
What are sphingomyelins and where are they found?
Sphingomyelins are sphingolipids with a phosphocholine head group. They resemble phosphatidylcholines in structure and have no net charge on their head group. They are abundant in plasma membranes of animal cells, especially in myelin sheaths that insulate neuron axons.
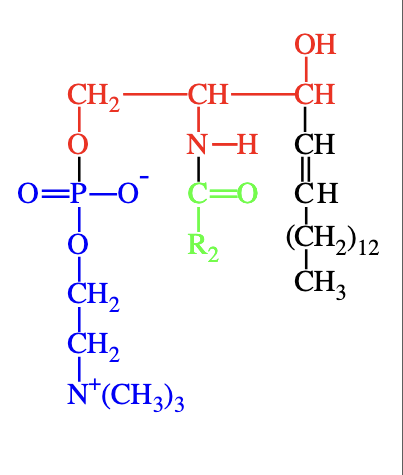
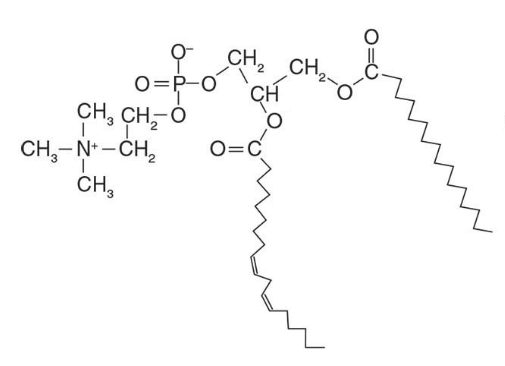
phophatidylcholine
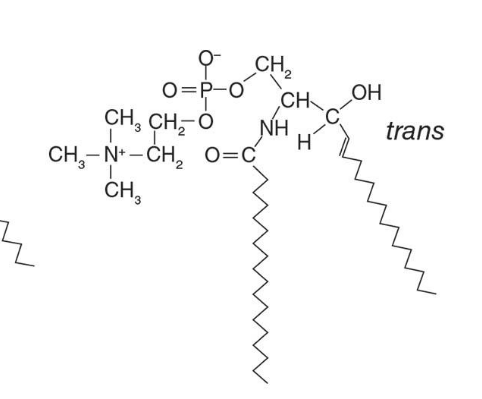
sphingomyelin