Chemistry: Gasses
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Pressure
a measurement of force per unit area
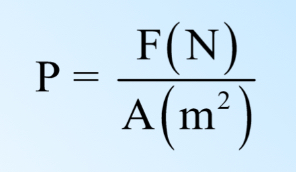
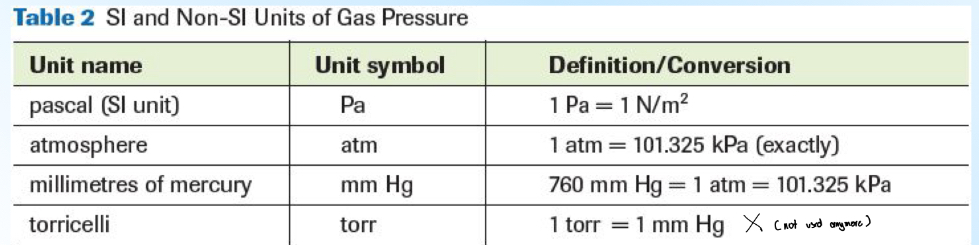
SI units / Non SI Units
torr is NOT needed to know
mmHg —> atm —> kPA
in order to find each, you must divide the x with the same x factor
example: 70 mmHg x 1 atm / 760 mmHg
atm —> kPa
kPA = atm x 101.325
atmospheric pressure
the force per unit area exerted by air on all projects
set at 101.325 kPA at sea level (in data booklet)
standard pressure - 760 mmHg = 1 atm = 101.325 kPa
Standard Temperature and Pressure
standard temperature and pressure to compare gas
1 atmosphere at 0c, OR 101.325 kPa at 273.15K
Standard Ambient Temperature and Pressure (SATP)
100 kPa at 25c (ROOM TEMPERATURE!)
OR 100 kPa at 298.15K

LALALA.
Pressure and Volume
As the pressure in a gas can increases, the volume decreases
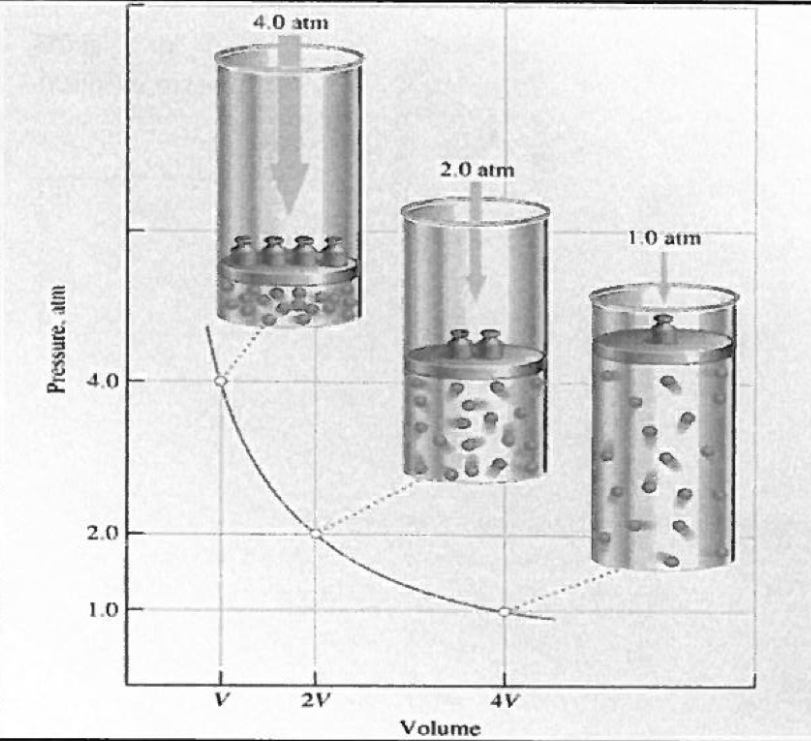
Boyle’s Law
As the pressure on a gas increases, the volume on the gas decreases proportionally, provided that the temperature and chemical amount remain constant
PV = k
P1V1 = P2V2
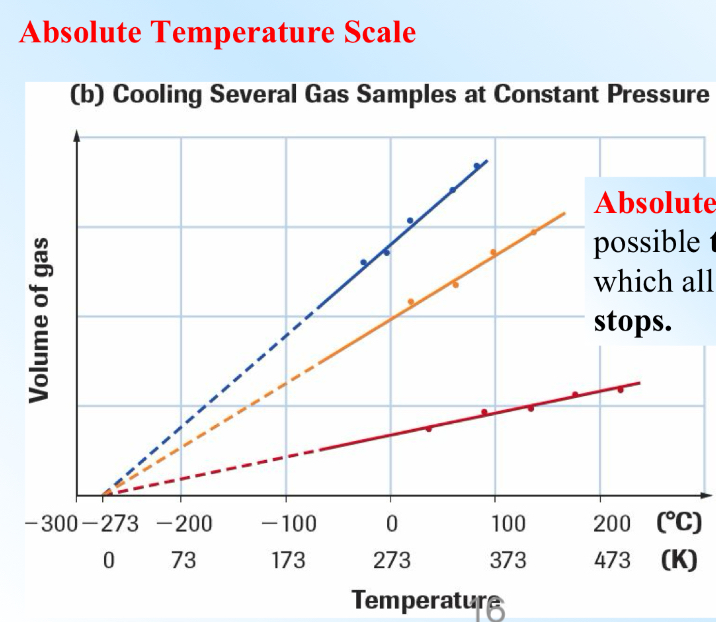
Absolute Temperature Scale / Kelvin
-273C
This is the lowest possible temperature at where all molecular motion stops.
STP/SATP
STP (Standard Temperature)
273.15K and 101 kPa
SATP (Stanrdard Ambient Temperature)
298.15K and 100kPa
Charles’ Law
As the temperature of a gas increases, the volume increases, provided that the pressure and chemical amount of gas remains constant.

Kelvin VS Celsius + Example
Kelvin Temperature: Must add 273 with a K!
Celsius Temperature: Must subtract 273 with a K!

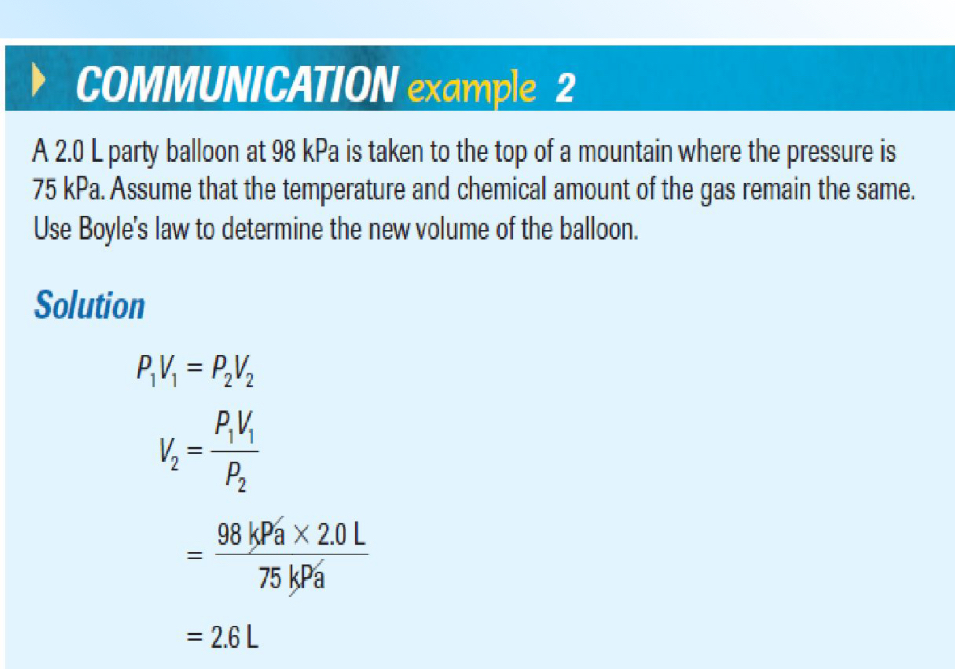
The Combined Gas Law
The product of the pressure and volume of gas sample is proportional to its absolute temperature in kelvin. PV = kT
Pressure/Chemical Amount cannot change
Boyle’s Law —> PV = k
Charles’ Law —> V/T = k
PV/T = K
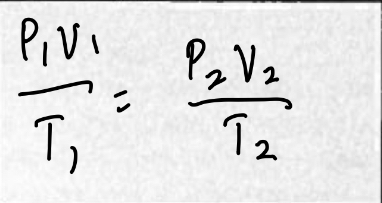
The Combined Gas Law Example
Set/fixed are the same, so you can cross them out
Add units!
Postulates of the Kinetic Molecular Theory of Gases
Gases are made out of tiny particles
Particles are so small, they can be assumed to be zero
Particles in a constant motion, colliding against the wall, cause the pressure to be exerted
Particles don’t attract nor repel
Average kinetic energy of a gas particles is exact to the Kelvin temperature
Gas
total disorder, much empty space, particles have complete freedom of motion, particles far apart.
transnational form of motion
Liquid
Disorder, particles or clusters are free to move relative to each other, particles close..
Vibrational, rotational, and some transnational
Crystalline Solid
ordered arrangement, particles are in fixed positions; particles close together
vibrational nation
Gay-Lussac’s Law of Combining Volumes
when measured at the same temperature and pressure, volumes of reactants and products of chemical reactions are in simple ratios of whole numbers
2 H20 —> 2H2 + O2

Avogardo’s Theory
equal volumes of gases at the same temperature and pressure contain equal numbers!
coefficient, chemical amounts, volumes are all equal

Combining Values | Things To Note
Read the question carefully. Majority of times, it’s the first two, but you mus read carefully.
Example shown:
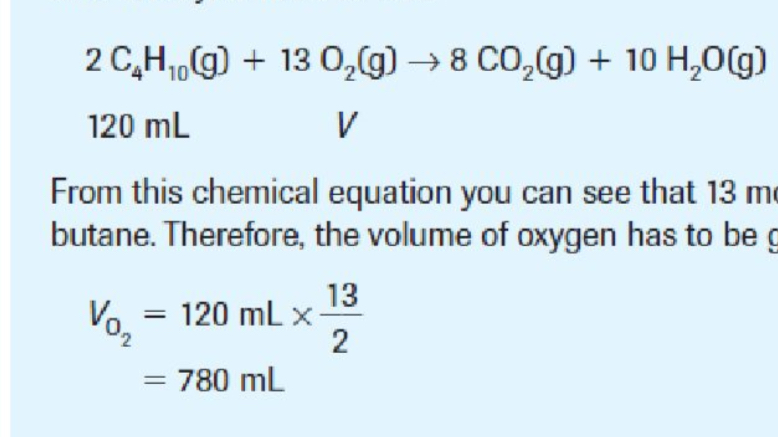
Molar volume
the volume that one mole of a gas occupies at a specfic temperature and pressure
as the temperature goes up, the volume will increase!
Molar volume — STP and SATP
STP:
23.15K and 101.325 kPA
Vm = 22.46 L/mol at STP
SATP
298.15K and 100 kPA
Vm = 24.8 L/mol at SATP
Molar volume | Remember
Elements matter to find your molar mass, you should always calculate it
Be sure to convert grams to molar, shown in the photo below
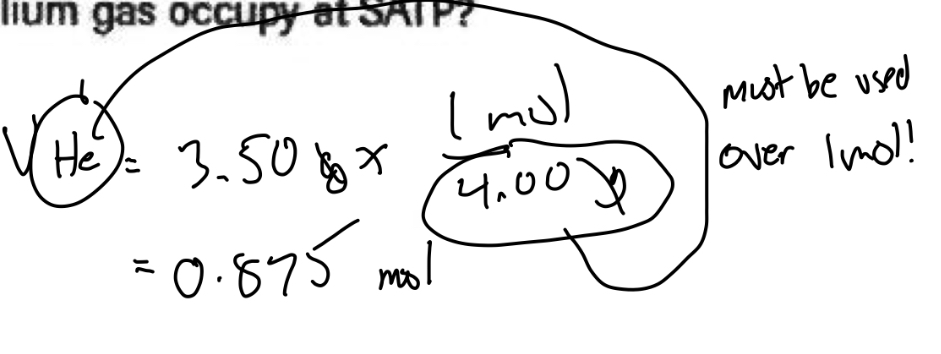
Ideal Gas
a hypothetical gas that obeys all gas laws perfectly
Kinetic Molecular Theory
gasses are very far apart compared to their size
gas molecules are constant, random, and straight line because no forces
molecules undergo perfect elastic collisions in no energy is lost and collisions occur quickly
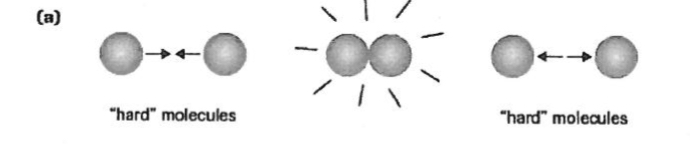
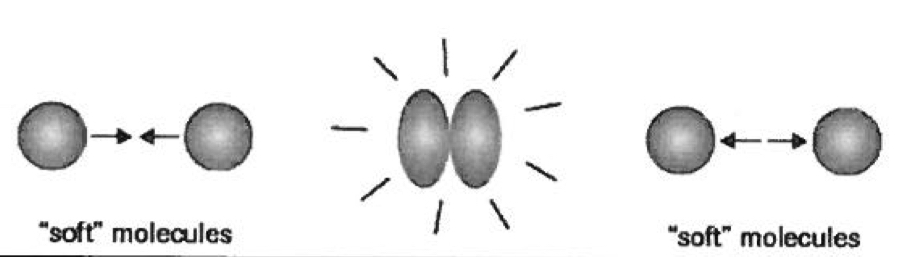
Real Gasses
high pressure — molecules are forced closer and size becomes significant
temperature decreases = molecules slow down.. at some point, it could stick together
molcules are more soft like, and change during collision. pressure is less then ideal..
Ideal Gas Law Formula
PV = nRT
P = Pressure (kPa) (/1000 if Pa)
V = Volume (L) (/1000 if L)
n = Chemical Amount (mol) (divide as so if g)
T = Temperature (must be K) (+273 to C)
R = universal gas constant (8.314 kPa x L / mol x K)