Organic Compounds
5.0(4)
5.0(4)
Card Sorting
1/18
Earn XP
Last updated 6:47 PM on 2/9/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
1
New cards
Organic Compounds
consists of carbon, hydrogen, oxygen, nitrogen.
2
New cards
Carbon
it can form single, double, triple bonds with another carbon or with other elements.
3
New cards
Functional Groups
groups of atoms that confer specific properties to a molecule
4
New cards
Hydrocarbons
organic compounds containing carbon and hydrogen
5
New cards
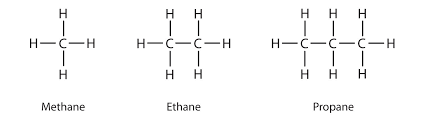
Alkanes
single bond between carbon atoms
6
New cards
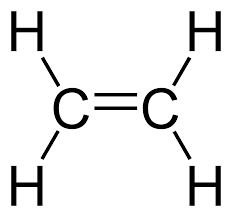
Alkenes
double bond between carbon atoms
7
New cards
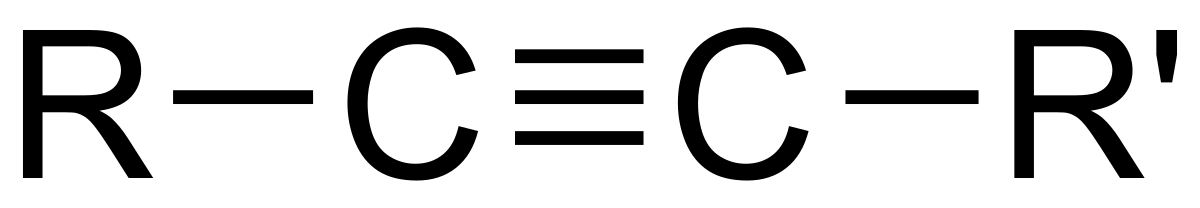
Alkynes
triple bond between carbon atoms
8
New cards
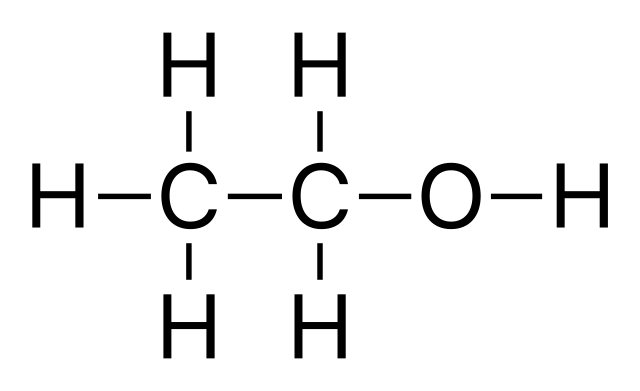
Alcohols
with an hydroxyl (-OH), that is bonded with an alkyl group (R),
(R-OH).
(R-OH).
9
New cards
Ethers
An oxygen (O) between two alkyl groups (R).
(R-O-R)
(R-O-R)
10
New cards
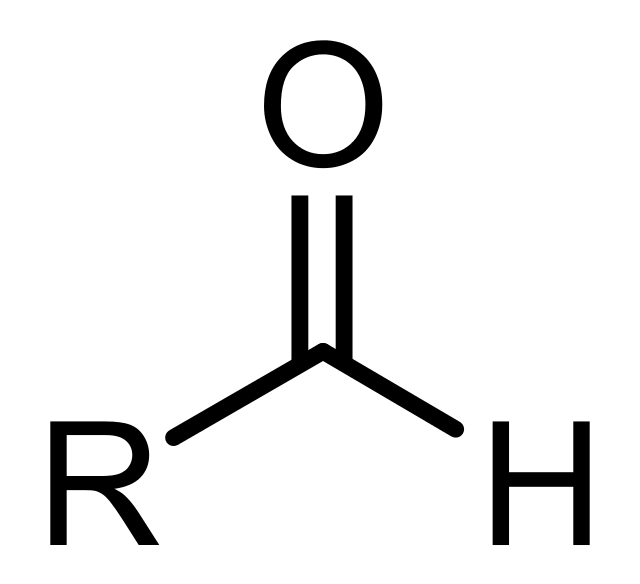
Aldehydes
consists of a carbonyl group (C=O)
which is bonded to one H and an R group.
which is bonded to one H and an R group.
11
New cards
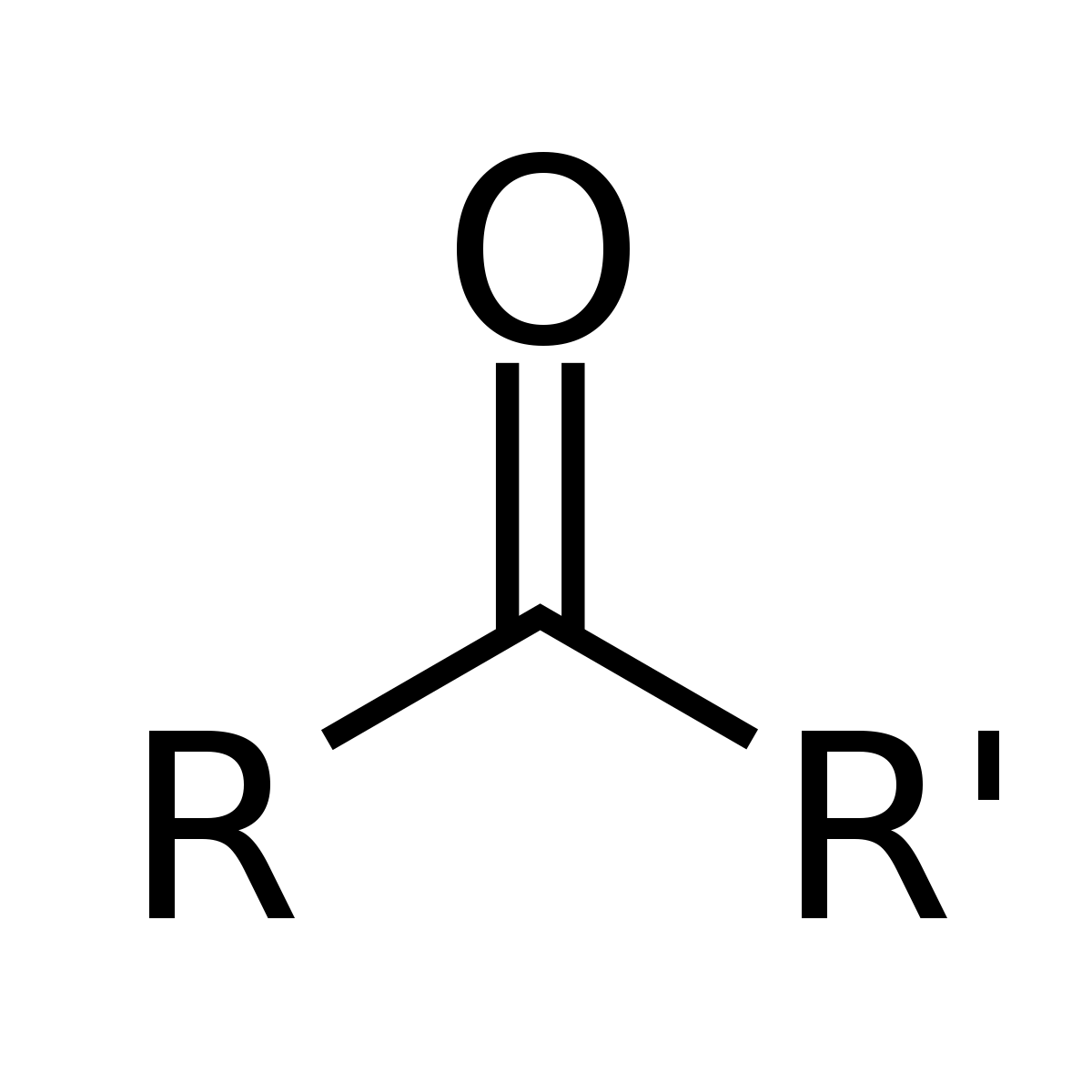
Ketones
has a carbonyl (C=O) group attached to two hydrocarbon groups (R)
12
New cards
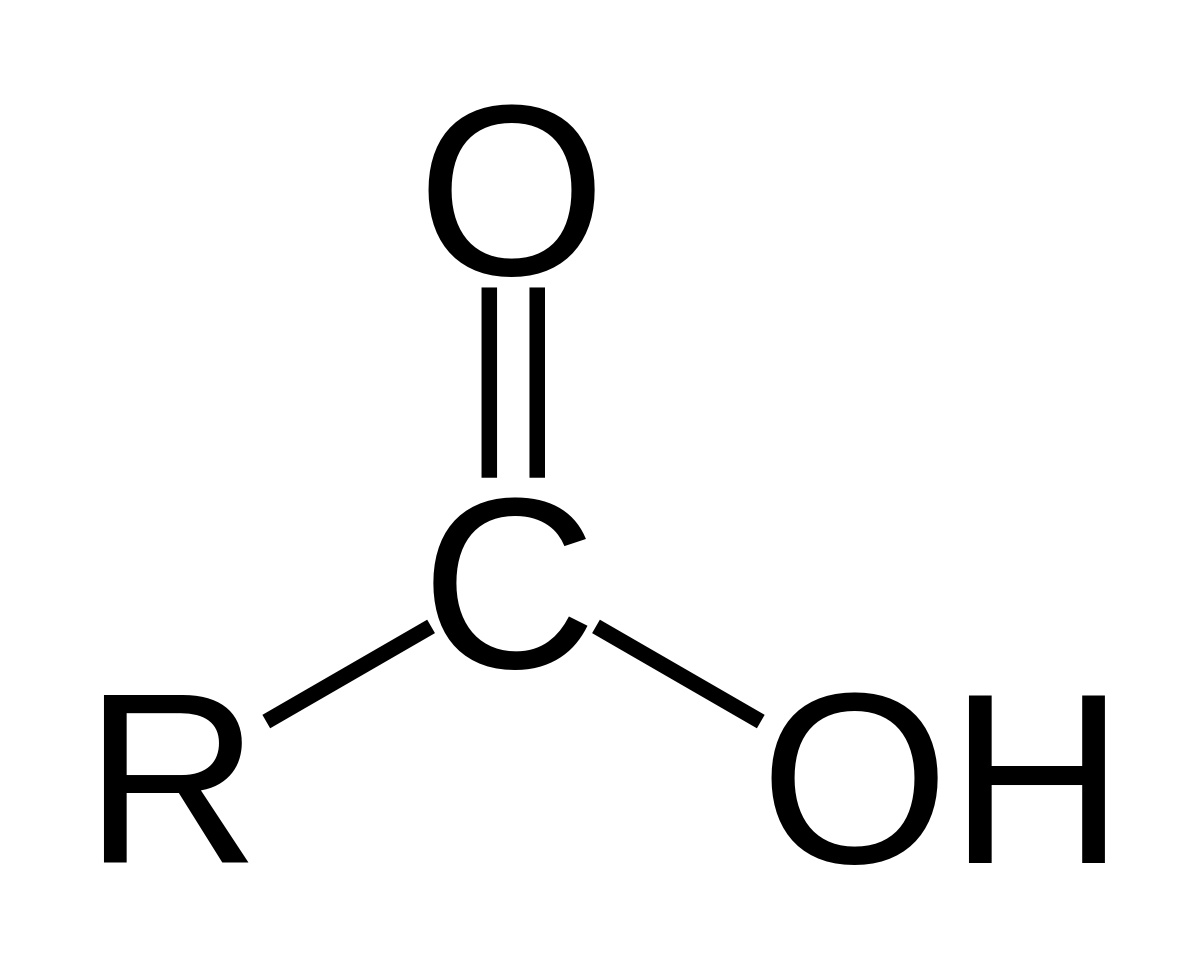
Carboxylic Acids
consists of carbonyl (C=O) and hydroxyl (OH) groups
13
New cards
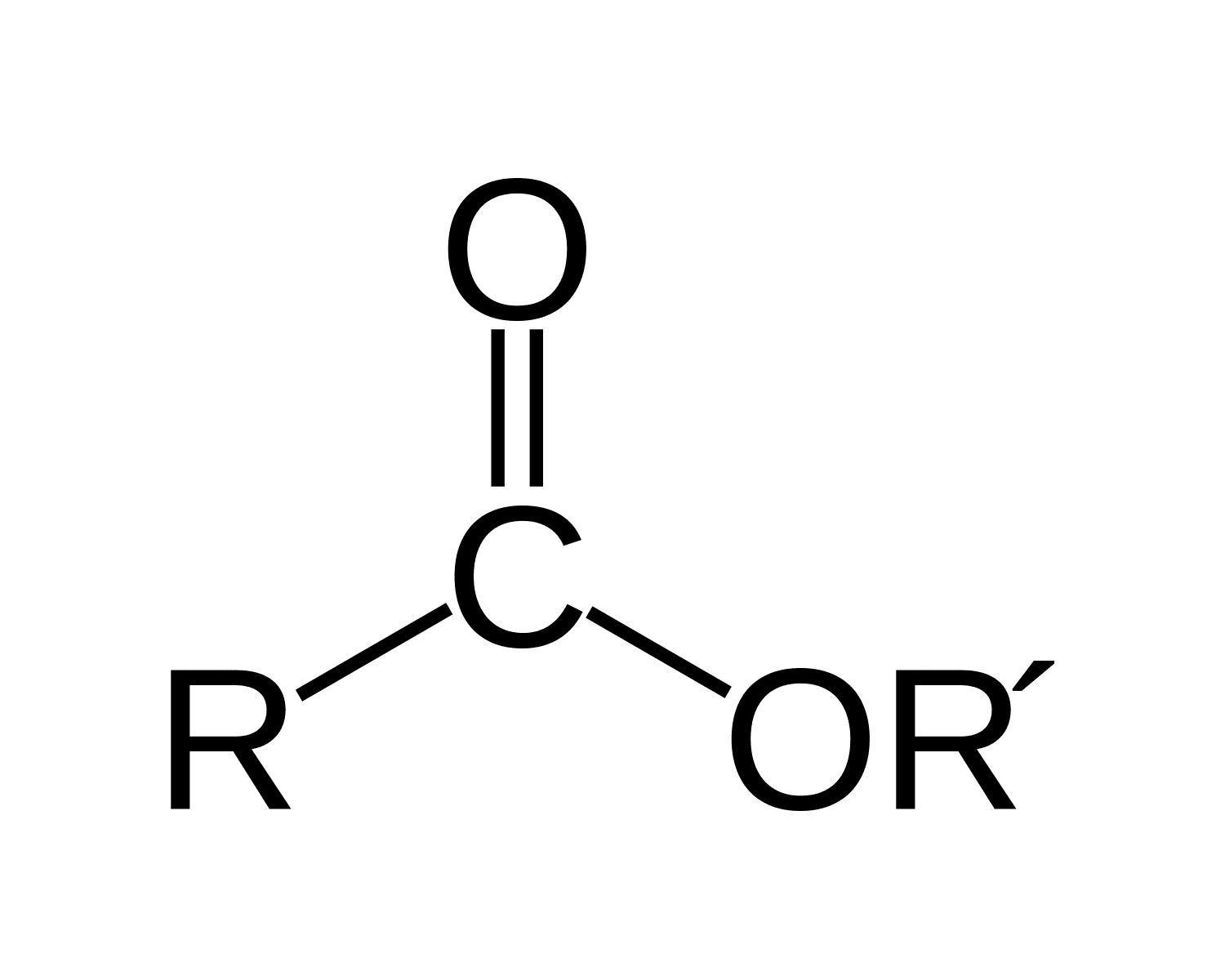
Esters
consists of a carbonyl group (C=O)
adjacent to an ether linkage (-OR)
adjacent to an ether linkage (-OR)
14
New cards
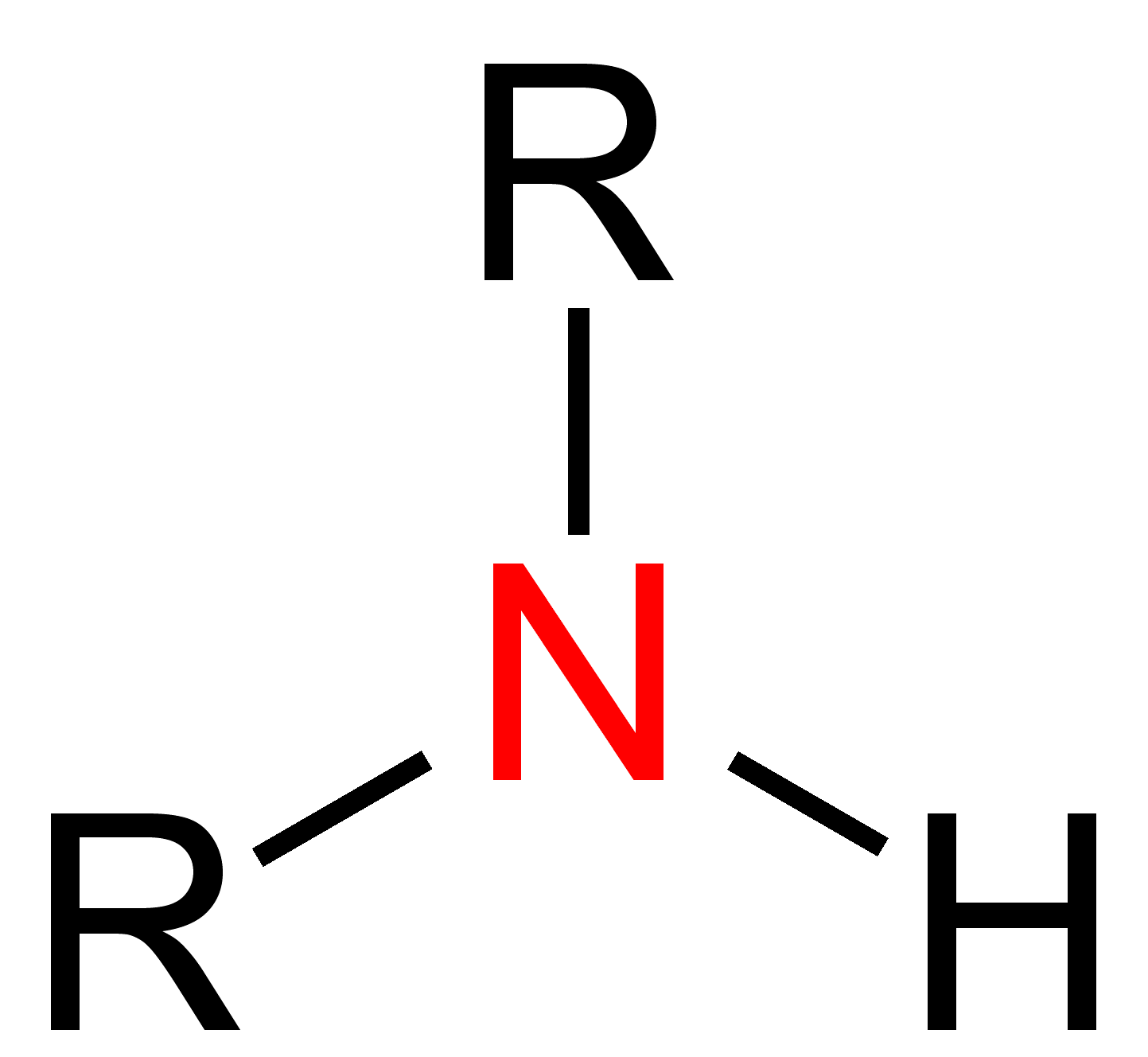
Amines
Derivatives of ammonia (NH3) where in one or more hydrogen atoms have been replaced by an alkyl group.
(R-NH2)
(R-NH2)
15
New cards
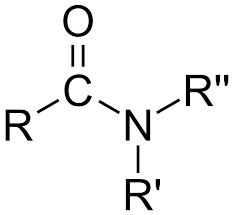
Amides
carboxylic acid with an amine.
Carbonyl group (C=O) linked to a nitrogen (N)
Carbonyl group (C=O) linked to a nitrogen (N)
16
New cards
Hydroxyl Group
\-OH
17
New cards
Carbonyl Group
C=O
18
New cards
Ether Linkage
\-OR
19
New cards
Carboxyl Group
C=O + OH