MCAT General Chemistry - COMPLETE
1/492
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
493 Terms
Nucleus
centre of atom, holds most mass, protons and neutrons
fundamental unit of charge (e)
magnitude of charge of a proton or electron (e = 1.6 × 10-19 C)
atomic mass unit (amu)
mass of one proton or neutron, exactly 1/12 the mass of carbon-12
Proton
subatomic particle with charge of +1 e, mass = 1 amu, found in the nucleus
atomic number (Z)
identifies element, number of protons in one atom
neutron
subatomic particle with no charge of , mass > 1 amu, found in the nucleus
mass number (A)
sum of the protons and neutrons in one atom
Isotopes
Atoms that have the same atomic number but different mass numbers; have the same number of protons but varying numbers of neutrons; referred to by the name of element followed by mass number; same atomic number means similar chemical properties
Electrons
subatomic particle with charge of - 1 e, mass = 1/2000 amu (often considered 0), found outside the nucleus
Electron shell
a given distance from the nucleus, corresponding to a particular level of electrical potential energy
Valence electrons
Electrons furthest from the nucleus; strongest interactions from surrounding environment and weakest with nucleus; involved with bonding
Ion
charged atom
Cation
positively charged ion
Anion
negatively charged ion
Hydrogen Isotope Names
protium : Z=1, A=1
deuterium : Z=1, A=2
tritium : Z=1, A=3
Atomic weight
weighted average of naturally-occurring isotopes of an element; represents mass of ‘average’ atom in amu and mass of one mol of element in g.
Mole (mol)
number of things equal to Avogadro’s number
Avogadro’s number (NA)
6.02 × 1023
Ernest Futherford
1910 - proved atom has small, dense, positively charged nucleus - gold foil experiment
Max Planck
1899 - first quantum theory, energy emitted as electromagnetic radiation comes in discrete bundles/quanta - blackbody experiments/ultraviolet catastrophe
Quantum (pl. quanta)
discrete amount of energy
Planck relation
relates the energy of a quantum to frequency via a proportionality constant; E=hf=hν=hc/λ
Planck’s constant (h)
proportionality constant, 6.626 × 10−34 J · s
frequency (f/ν)
wave frequency of radiation
Niels Bohr
1913 - developed planetary model of atom; quantized angular momentum
angular momentum (L)
a vector quantity that describes the rotary inertia of an object or system; L=mvr=nh/2π
Rydberg equation
E= - RH/n2= RH[1/ni2-1/nf2]; negative = attractive force towards nucleus; energy of electron increases at increasing n
Rydberg unit of energy
2.18 × 10−18 J/electron
orbit
defined pathway of an electron at a discrete energy level
ground state
state of lowest energy; all electrons are in lowest possible orbitals
excited state
at least one electron is at a higher energy level
Atomic Emission Spectrum
spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a high energy state to a lower energy state; each element has unique set of energy levels
line spectrum
representation of atomic emission spectra, where each line represents light at a specific frequency
Lyman series
hydrogen emission lines from n ≥ 2 to n = 1
Balmer series
hydrogen emission lines from n ≥ 3 to n = 2
Paschen series
hydrogen emission lines from n ≥ 4 to n = 3
Atomic Absorption Spectrum
the fraction of incident radiation absorbed by the material over a range of frequencies of electromagnetic radiation; electrons absorb specific amounts of energy to get excited; equal to emission wavelengths
orbitals
regions of space where electrons are often localised, holds two electrons of opposite spins
Heisenberg Uncertainty Principle
It is impossible to simultaneously determine, with perfect accuracy, the momentum and the position of an electron.
Quantum numbers
numbers that describe electrons in an atome
Pauli Exclusion Principle
No two electrons in a given atom can possess the same set of four quantum numbers.
Principal quantum number (n)
represents energy level/electron shell, any positive integer value, max # electrons in shell - 2n2
azimuthal (angular momentum) quantum number (l)
shape and number of subshells within given shell; integers btwn 0 and n-1, max # electrons in subshell = 4l+2
Spectroscopic notation
shorthand representation of the principal and azimuthal quantum numbers; l=0=s, l=1=p, l=2=d, l=3=f
magnetic quantum number (ml)
specifies orbital; integers from -l to l, including 0; 2 electrons per orbital
spin quantum number (ms)
specifies spin orientation, ±½
paired electrons
two electrons that occupy the same orbital and have opposite spins
parallel spin
electrons in different orbitals but same spin number
Electron Configuration
the pattern by which subshells are filled, as well as the number of electrons within each principal energy level and subshell; can be abbreviated by placing the noble gas that precedes the element of interest in brackets
Aufbau (building-up) principle
Electrons fill from lower- to higher-energy subshells
n + l rule
the lower the sum of the values of the first and second quantum numbers, n + l, the lower the energy of the subshell.
Hund’s Rule
within a given subshell, orbitals are filled such that there are a maximum number of half-filled orbitals with parallel spins; due to electron repulsion; half-filled and fully filled orbitals have lower energies (higher stability) than other states
paramagnetic
Materials composed of atoms with unpaired electrons will orient their spins in alignment with a magnetic field, and the material will thus be weakly attracted to the magnetic field
diamagnetic
Materials consisting of atoms that have only paired electrons will be slightly repelled by a magnetic field
Periodic Table of the Elements (PT)
ordering of the known elements by atomic weights/number; reveals a pattern of periodically recurring physical/chemical properties
periodic law
the chemical and physical properties of the elements are dependent, in a periodic way, upon their atomic numbers
periods
rows of PT; 7 representing the first 7 principal quantum numbers; each element in a given period has one more proton and one more electron than the element to its left
groups/families
columns of PT; Groups contain elements that have the same electronic configuration in their valence shell and share similar chemical properties
may be named by Roman numeral of valence electrons and split into A/B classes OR 1-18 (modern IUPAC stanadard)
valence shell
outermost shell of electrons
valence electrons
The electrons in the valence shell, farthest from the nucleus and having the greatest amount of potential energy; can form bonds with valence electrons of other atoms
A/representative elements
valence electrons in s or p subshells
IA - VIIIA
B/nonrepresentative elements
include both the transition elements and the lanthanide and actinide series; may have unexpected electron configurations
transition elements
have valence electrons in the s and d subshells; can have multiple oxidation states due to losing different numbers of s and d electrons
(Groups IB-VIIIB/3-12)
some (Cu, Ni, Ag, Au, Pd, Pt) are particularly nonreactive
lanthanide and actinide series
have valence electrons in the s and f subshells
metals
lustrous solids* with high melting points, high densities^; can be deformed without making; good conductors
low effective nuclear charge, low electronegativity (high electropositivity), large atomic radius, small ionic radius, low ionisation energy, low electron affinity
left and middle of PT
*except mercury (liquid @ RT)
^excpet lithium (half that of water)
lustrous
shiny
malleability
ability of metal to be hammered into shapes
ductility
ability to be pulled or drawn into wires
oxidation states
charges when forming bonds with other atoms
conductor
can transmit heat and electricity
i.e. metals are good conductors because of their loose, free-moving valence electrons
active metals
valence electrons found in s subshell
do not exist naturally in neutral forms; always found in ionic compounds/minerals/ores
nonmetals
brittle and dull when solid; poor conductors; less unified in chemical and physical properties than metals
high electronegativity (low electropositivity), small atomic radius, large ionic radius, high ionisation energy, high electron affinity
upper right of PT
*except mercury (liquid @ RT)
^excpet lithium (half that of water)
metalloids/semimetals
physical properties vary widely
chemical properties between metals and nonmetals; reactivities depend on elements with which they are reacting
staircase btwn metals and non-metals (B, Si, Ge, As, Sb, Te, Po, At)
effective nuclear charge (Zeff)
electrostatic attraction between the valence shell electrons and the nucleus
increases along period; largely stable along group
noble (inert) gases
have a full octet; minimal chemical reactivity; high ionisation energies and no measurable electronegativities
Group VIIIA/18
atomic radius
equal to one-half of the distance between the centers of two atoms of an element that are briefly in contact with each other
decreases along period; increases along group
ionic radii
metals lose electrons and become positive, while nonmetals gain electrons and become negative
metalloids can go in either direction, but tend to follow the trend based on which side of the metalloid line they fall on. (Si behaves more like a nonmetal, while (Ge) tends to act more like a metal)
non-metal ions closest to metalloid staircase are largest
metal ions closest to metalloid staircase are smallest
Ionization energy (IE)/ionization potential
energy required to remove an electron from a gaseous species
subsequent removals will require increasing amounts of energy
increases along a period, decreases along a group
Electron affinity
to the energy dissipated by a gaseous species when it gains an electron (reported as a positive number)
increases along a period; decreases along group
Electronegativity
measure of the attractive force that an atom will exert on an electron in a chemical bond
increases along a period; decreases along group
Alkali metals
possess most of the classic physical properties of metals, except that their densities are lower than those of other metals; only one loosely bound electron in their outermost shells - form monovalent cations
Group IA/1
Alkaline earth metals
possess most of the classic physical properties of metals; two electrons in their outermost shells - form divalent cations
Group IIA/2
Chalcogens
eclectic group of nonmetals and metalloids; some are crucial for normal biological functions, the rest are metallic and toxic, and all are toxic in high doses
Group VIA/16
Halogens
highly reactive nonmetals with seven valence electrons; multiple states of matter; so reactive only found as ions or diatomic molecules
Group VIIA/17
halide
anion of halogens
hydration complexes
complex ions interactions with water
compex ions
Any ion in which a central metal atom (normally a transition element) is surrounded by a group of ions or molecules (ligands)
known for varied bright colors; absorbs certain frequencies of light
subtraction frequencies
light reflected or not absorbed by an object that gives the object its color
complementary color to the frequency that was absorbed
complementary color
‘opposite’ colour, as in a colour wheel
Maillard reaction
a nucleophilic reaction between the amino terminus of the peptide chain of a protein and the carbonyl functionality of a sugar to form an N-substituted glycosylamine; a complex series of rearrangements and other reactions to produce a set of compounds that gives cooked food its pleasing color and delectable flavor
ex. browning meat, crisping cookies
molecules
combinations of bonded atoms
chemical bonds
strong attractive forces between atoms in a molecule formed via the interaction of the valence electrons of the combining atoms
octet rule
an atom tends to bond with other atoms so that it has eight electrons in its outermost shell, thereby forming a stable electron configuration similar to that of the noble gases
Incomplete octet
stable with fewer than 8 electrons in their valence shell
hydrogen (2 electrons)
helium (2)
lithium (2)
beryllium (4)
boron (6)
Expanded octet
Any element in period 3 and greater can hold more than 8 electrons
phosphorus (10)
sulfur (12)
chlorine (14)
Odd numbers of electrons
Any molecule with an odd number of valence electrons cannot distribute those electrons to give eight to each atom
ex. nitric oxide (NO) has eleven valence electrons
common elements that almost always abide by the octet rule
carbon, nitrogen, oxygen, fluorine, sodium, magnesium
ionic bonding
one or more electrons from an atom with a low ionization energy, typically a metal, are transferred to an atom with a high electron affinity, typically a nonmetal; difference in electronegativity must be greater than 1.7 on the Pauling scale

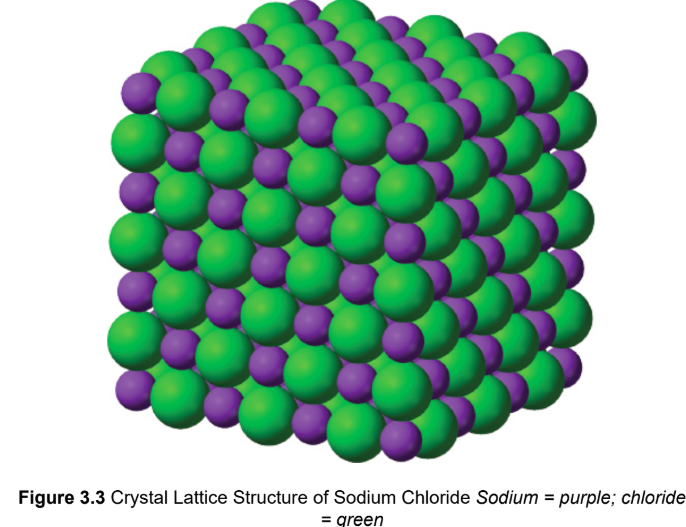
ionic crsytalline lattice
repeating rows of cations and anions; attractive forces between oppositely charged ions are maximized, and the repulsive forces between ions of like charge are minimized
covalent bonding
an electron pair is shared between two atoms, typically nonmetals, that have relatively similar values of electronegativity