Atmospheric Chemistry Extracted
1/150
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
151 Terms
1970 air quality standards for five(later six) air pollutants
PM - Suspended particles/particulate matter
respirable particulate matter PM10 (size < 10 um)
fine particulate PM2.5 (size < 2.5 um)
SO2 - sulfur dioxide
CO - carbon monoxide
NOx - nitrogen oxides
O3 - Ozone
Pb - Lead (added later)
Total Ozone Mapping Spectrometer (TOMS)
Measures the total atmospheric ozone column by measuring UV region, at six wavelengths between 213 and 380 nm, in which few other atmospheric molecules absorb strongly
Suspended particulate matter
Natural sources of airborne particles, solid or liquid, including smoke, dust, sea, salt spray, pollen grains, bacteria, fungal spores
Liquid aerosols and particulates that are liquid are called mist, including fog and raindrops
Sources of CO
Anthropogenic sources of CO
combustion of gasoline in confined ICE
2C8H18 + 17O2 = 18H2O + 16CO
combustion by electric power plants
industrial processes
solid waste disposal
natural sources (10 times more CO than all anthropogenic sources combined)
methane gas (released during anaerobic decay of plant materials in swamps, rice paddies, wetlands where vegetation is in oxygen-depleted water)
methane from cattle/sheep termites
food digestion produces methane, flows to bloodstream, released during exhale
methane oxidizes to CO
2CH4 + 3O2 = 2CO + 4H2O
mechanisms to maintain average global level constant at 0.1 ppm CO
conversion of CO to CO2 in hydroxyl radical reactions
removal of CO from atmosphere by microorganisms in soil
total suspended particles (TSPs) in cities and effects
TSPs up to 200 ug m-3 have been measured in large cities
Individual measurements as high as 500 ug m-3 measured in dusty locations
Rural farming areas average 10g to 50 ug m-3
Chlorofluorocarbons (CFC) pollution
Besides refrigeration, CFCs are released directly into atmosphere
Tetraethyl lead, which was invented, is the octane-increasing additive for gasoline
Leaded gasoline partly responsible for global lead pollution
Commercially, the most important CFCs are halogenated methane Freon 11 and Freon 12
Tropopause
Top of troposphere
Low temperature (-57C) serves as barrier that freezes water vapor as ice crystals
Lithification
sedimentary rock formation
primary air pollutants
CO - carbon monoxide
SO2 - sulfur dioxide
NOx - nitrogen oxides
VOCs - volatile organic compounds (mostly hydrocarbons HC)
Suspended particles
Makes up approximately 90% of all air pollution in the US
fluidized bed combustion FBC
Process in which mixture of pulverized coal and powdered limestone burned, with air being introduced to keep mixture in semifluid state
Limestone converted to CaSO4,
Coal finely divided, so reaction occurs at lower temperature than in flue-gas desulfurization FGD. As result, NOx emissions lower
Disadvantage of FBC is it cannot be added to power plants, but is preferred technology for new power plants
Both FGD and FBS have problem of disposing large quantities of CaSO4
methane
25 times more effective than carbon dioxide at trapping heat
steady rise attributed to increase in cattle and rice paddies
is polyatomic molecule with tropospheric concentration of 1.75 ppmb and increasing at a rate of 0.5% per year
Methane absorbs IR in 3 to 4 μm (not important because water and CO2 already absorbed) and 7 to 8.5 μm (within atmospheric window, can contribute to greenhouse effect)
settling rate example: calculate settling rate for particulate of fly ash that is 2 μm in diameter and has density of 1.0 g/ml
Convert density into g/m3
Plug values into equation
Solve for settling rate (distance dropped in a specific unit of time)
Upper atmosphere
Extends beyond stratosphere
Divided into mesosphere and thermosphere
primary organic aerosols (POA)
organic particulate matter emitted directly as particles
CFC Substitutes
HCFCs (hydrochlorofluorocarbons) have less chlorine atoms nd break down more readily in troposphere, but cause ozone destruction
better substitute HFCs (hydrofluorocarbons), no chlorine. used in car ACs. can still contribute to climate change
Earth reradiates EMR as
infrared
Average wavelength of this radiating EMR, which is 10 um, which corresponds to the emission line for a 300 K black body emitter
NOx effects on human health
NO2 is a red-brown toxic gas with unpleasant acrid odor
causes eye irritation, inflammation of lung tissue, and emphysema
concentration in atmosphere not high enough to produce symptoms
NOx is serious because of role in formation of secondary pollutants associated with photochemical smog
lowering combustion temperature of furnace decreases formation of NO, but decreases efficiency
Infrared absorption and molecule vibrations
Infrared radiation not energetic enough to break covalent bonds or cause electronic transition, but can change vibrational or rotational motion of a molecule
To absorb infrared radiation, molecule must undergo a net change in dipole moment as a result of vibrational motion or rotational motion around covalent bond
Because molecules are symmetrical, no matter how much covalent bonds stretched, no change in dipole moment
Therefore, these molecules which are principal constituents of the atmosphere, cannot absorb infrared radiation.
At altitude 6 km, atmospheric pressure reduced to
50% of value at sea level
Average value used is 760 torr or 101,325 Pa
soot
Finely divided, impure form of carbon in which structure contains a series of fused benzene rings
Particles are spherical, whereas graphite forms flat layered structure
Product of incomplete combustion of coal
earth’s albedo
solar flux that is reflected back into space (31%)
percentages of solar flux absorption and reflection
Only approximately 69% of total solar radiation or solar flux reach Earth is absorbed. Remaining 31% reflected back into space
Of the 69% absorbed, 23% absorbed by water droplets in clouds and other gaseous molecules such as ozone in atmosphere
Remaining 46% absorbed and used for energy sources for biomass and thermal warming
Stefan Boltzmann Law
Energy reradiating from entire area of Earth’s surface Eout =
Steady-state total energy Earth absorbs from sun
Allows predicting average global temperature of -19C, but measurement is up to 15C
Calculation assumes that all radiation leaves Earth and is lost in space
If some IR radiation is absorbed by gases in the atmosphere and not lost, then planet’s temperature will be higher
Fly ash collection
Industry collects vast majority with electrostatic precipitation or bag houses
Majority of products used in construction-related applications, including cement and concrete, structural fills, soil stabilization, stabilization of waste, mineral filler in asphalt
Beer-Lambert/Beer’s Law
Relationship between the concentration of the absorbing molecule and absorbance (dimensionless)
Pathlength (b) can be very small (cm) or very large (km)
Quantity or molar absorptivity or extinction coefficient (ε), is characteristic of a molecule that indicates how much light it will absorb at a particular wavelength
Adsorption
if impacting molecules, become attached to the particle’s surface
Absorption
in the case of liquid particles, molecules are drawn inside and dissolved
Purpose of atmosphere
Shields the Earth’s surface from sun’s cancer causing UV radiation and moderates Earth’s climate
Stratified due to temperature and density relationships resulted from the interaction of physical and photochemical processes (induced by sun light)
Rayleigh-scattering
Light scattering by aerosols with dimensions that are significantly smaller than wavelength of radiation
Intensity is proportional to the inverse fourth power of the wavelength (S = 1/λ4)
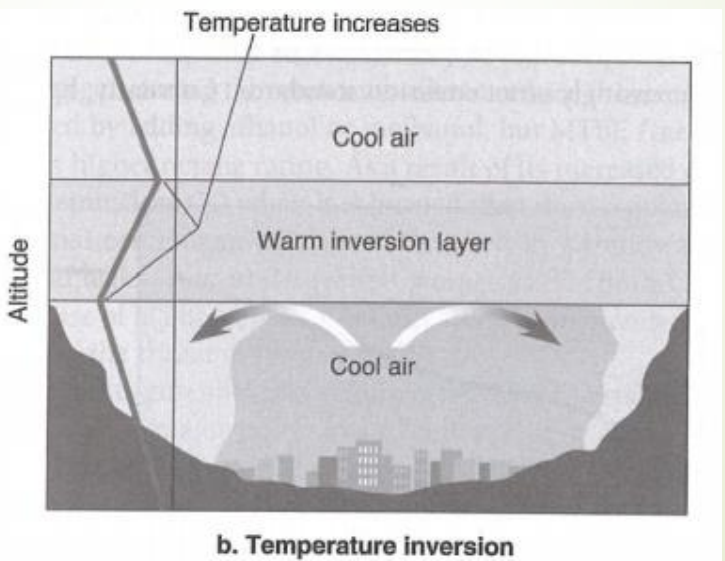
temperature inversion
reversal of the usual temperature pattern
air temperature begins to increase instead of decrease with increasing altitude
colder denser layer cannot rise through warm lid of air and is trapped
no vertical circulation, so pollutants accumulate
nitric oxide measurements (chemiluniscene)
chemical reactions that release energy produced by emitting light rather than heat (fireflies)
NO in tailpipe emissions reacted with ozone
Two-step process.
Ozone reacts with NO to produce excited-state nitrogen dioxide molecule (NO2). electrons in NO2 not in nlowest energy state but are in excited state
NO2 loses excess energy as the excited electrons return to ground state and emit excess energy as a photon of light
increase in atmospheric carbon dioxide
20,000 years ago, concentration was approximately 200 ppm
Before industrial revolution at end of 19th century, 280 ppm
1958 measurements at Mauna Loa, 315 ppm
2000 measurements at Mauna Loa, 370 ppm
30% increase in 100 years, mostly caused by burning of fossil fuels by electric facilities, automobiles, industry
null cycles
Processes that prevent certain species from taking part in catalytic cycles
in situ ozone measurements
Ultraviolet absorption used to measure concentration of ozone in stratospheric air
Other atmospheric gases (O2, N2 and H2O) do not absorb UV radiation so do not have to be removed from air sample since they do not interfere with ozone measurement
Concentration of ozone in stratosphere is less than 10 ppm, so spectrophotometer must be capable of measuring small absorbance
Beer’s law states absorbance proportional to sample pathlength and because obtaining sample is easy, long sample pathlength greatly improves ability to measure ppm
XRF process
Incident x-rays cause sample to release photons. Emitted photons released from sample are observed 90 degrees to incident x-ray beam. Collects all photons simultaneously
Each photon strikes silicon waver treated with lithium and generates electrical pulse proportional to energy of photon
Pulse height proportional to energy of photon
Concentration of element determined by counting pulse number
nitric oxide journey up
Cars and truck engines release large quantities of nitric oxide (NO) into troposphere
Almost all NO is oxidized to NO2 then converted to nitric acid HNO3
Rainfall washes nitric acid from troposphere before reaching stratosphere
Nitrous oxide N2O is much less reactive than NO and eventually reaches stratosphere
N2O is also tailpipe pollutant, but also released by soil during denitrification by anaerobic bacteria
Above 30 km, N2O can absorb high-energy photons ton produce molecular nitrogen and excited oxygen atom O
humus
Soil sequester atmospheric CO2 by containment of partially decomposed organic matter
While total amount CO2 sequestered in soil unknown, improved soil conservation practices anticipated to help mitigate buildup of atmospheric CO2 at least slightly
controlling SO2 emissions
Sulfur removed from coal before combustion
SO2 removed from smokestack after combustion but before reaching atmosphere
flue-gas desulfurization (FGD)
Sulfur-containing compounds washed out (or scrubbed) by passing the chimney (flue) gases through slurry of water mixed with ground limestone (CaCO3) and or dolomite (CaMg(CO3)2) or both
On heating, calcium carbonate reacts with pacific SO2 and oxygen to form calcium sulfate (CaSO4)
Scrubber, which removes 90% of SO2 in flue gas, easily retrofitted to existing power plants
Percentage water vapor in atmosphere
0.1% to 5%, depending on temperature (vapor pressure of water increases with temperature), precipitation, rate of evaporation
Generally between 1% and 3% making it third most abundant constituent in air
Lowest temperature in atmosphere
-90C between 80-90 km elevation
Automobile pollutants
Motor vehicles are major source of CO, NOx, and volatile HCs
Cars emit 95% less pollutants than pre-1970 vehicles due to catalytic converters
NOx emissions difficult to reduce
NOx produced by four-cycle is near maximum at the ideal, stoichiometric air/fuel ratio
Lowering NO and HC can be done by two-stage combustion
Operate system rich in fuel
Operate system rich in air
Burns fuel completely, but not at high enough temperature to produce as much NOx
Stratified-charge engine uses this design to make most reductions in NOx emissions
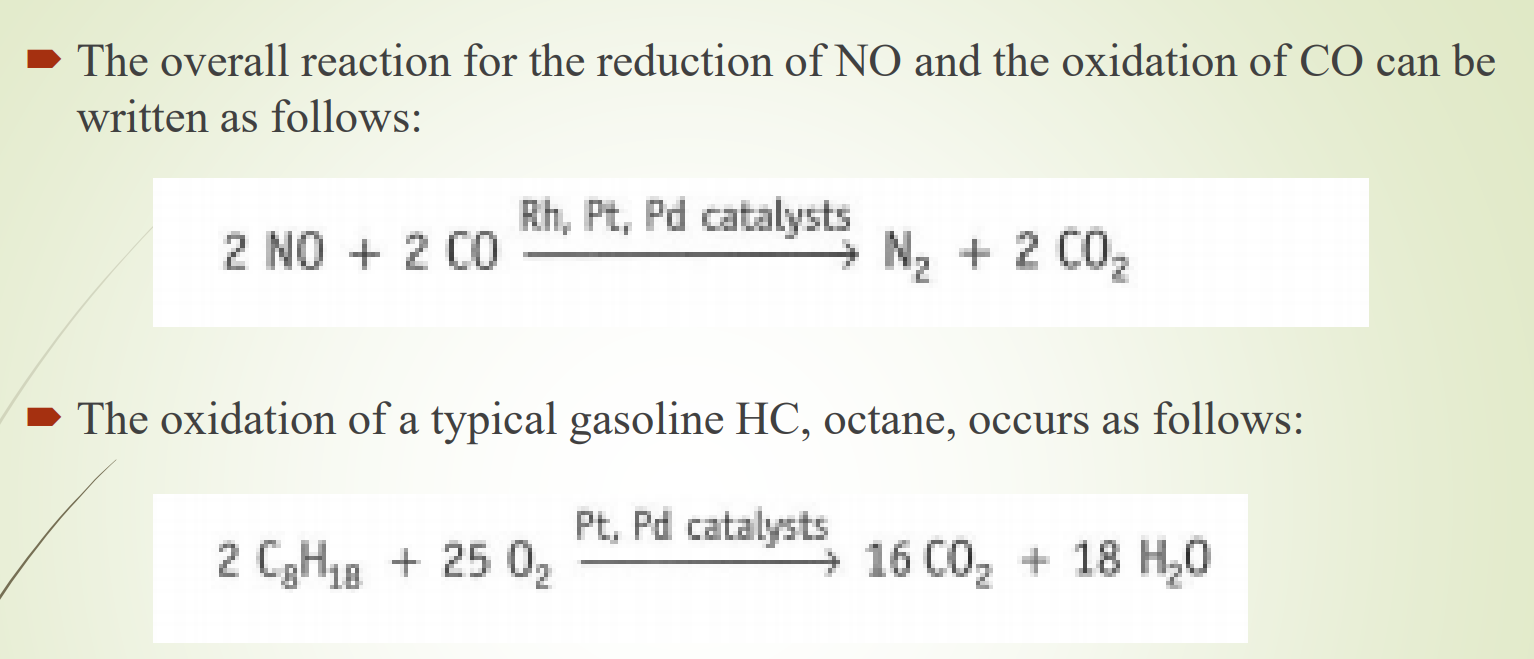
Three-way catalytic converter
Reduces amount of HC, NOx, and CO in exhaust stream
Very fine honeycomb structure made of ceramic coated with precious metals platinum (Pt), and rhodium (Rh) which act as catalysts
Forces CO emission to form CO2, and NO to form N2
air-fuel ratio needs to be set at 14.8:1
Atmospheric Trace Molecular Spectroscopy (ATMOS)
Has sun tracker that keeps instrument’s field of view on sun, and a telescope that collects IR radiation to be processed by spectrometer
Spectrometer simultaneously measures concentrations of gases in stratosphere between 10 and 150 km altitude
Composition of stratosphere can be determined because gas molecules absorb specific wavelengths of incoming solar radiation by determining the wavelengths that have been absorbed
Has ability to detect gases in concentrations lower than 1 ppb
Disadvantages: can only take measurements at sunrise and sunset
Overcome by measuring atmospheric emission rather than absorption
Most harmful particles
Very fine particles (diameters less than approximately 1 um) most hazardous to human health
Not filtered by hairs and mucus in nose but drawn deep into lungs, causing tissue damage and contributing to the development of emphysema
Aerodyne aerosol mass spectrometer (AMS)
Able to measure OA concentration and mass spectrum
Analysis of spectra suggested that OA even in heavily polluted area much more oxygenated than expected
Comparisons of hydrocarbon-like HOA mass spectrum with those of vehicle emissions led to strong association of HOA and POA
Analysis of AMS data collected at sites revealed oxygenated organic aerosol OOA component dominated everywhere
Earth’s heat balance
As solar radiation travels through atmosphere, interactions with gases and particulates prevent approximately half from penetrating to Earth’s surface
69% of solar energy reaching Earth includes entire visible region of spectrum with smaller portions of adjacent UV and IR regions
Incoming radiation largely absorbed at surface then reradiated back to space
If radiation away from surface did not occur, Earth would becoming increasingly warm as solar energy continued to flow in
Outgoing radiation from Earth is in longer wavelength IR region
Chlorine activation
In polar winter, chlorine-containing molecules accumulate
In spring, when sunlight returns to area, relatively inactive forms of chlorine in stratosphere such as ClONO2 and HCl converted to photochemically active forms, such as Cl2
Cl2 produced can go on to attack ozone
Free radicals
Uncharged fragments of molecules that have unpaired electrons
Highly reactive and short lived
Responsible for many of the reactions that occur in the normal and polluted atmosphere
Central to chemistry of the troposphere is hydroxyl radical *OH, which is uncharged and different from negatively charged OH-
Ground state
Lowest energy state of a molecule
Very high-energy electromagnetic radiation, such as X-ray
Has enough energy to break chemical bonds and ionize molecules
Irradiance
Intensity or radiant power
Energy per second per unit area of the light beam (W/m2s)
Electromagnetic radiation passed through a monochromator to select one wavelength of electromagnetic radiation
Monochromatic light, with irradiance P0, passes into sample of length b
Irradiance of beam emerging from other side of sample is P
Some light may be absorbed by sample, thus p<=0
Stocks law
For particles in which the diameter exceeds 1um, the settling velocity V
Atmospheric window
Unobstructed region of spectrum between 7.5 and 13 um, through which infrared radiation from Earth’s surface can still escape
Carbon Dioxide on Earth
Combined infrared absorption of carbon dioxide and water coincide with most of the infrared emission from Earth
Emissions from the burning of fossil fuels primary anthropogenic source of CO2
Carbon store in fossil fuels sequestered for millions of years beneath surface un release through combustion process
Natural sources of atmospheric co2 include decomposition of organisms, plant and animal respiration, and ocean processes
Several important CO2 sinks, which are mechanisms that remove CO2 from the atmosphere operate on plant Earth
LIMS
limb infrared monitor of the stratosphere
Nimbus 7 satellite 1978, used to measure IR emissions of the Earth
Polar-orbiting satellite able to complete a picture of the entire stratosphere in one day
Radiance of thermal emission of Earth’s atmosphere much less than sun, therefore necessary to use broader spectral bands for measurement rather than a high-resolution spectrum
IR radiation from five spectral bands measured, enabling LIMS to measure global distribution of trace gases (CO2, O3, H2O, HNO3-, NO2)
Fly ash from coal-fired boilers
Produces approximately 1% to 2% fly ash, with diameter 0.1um
Accounts for largest number of particles and most surface area
Contains Fe, Zn, Pb, V, Mn, Cr, Cu, Ni, As, Co, Cd, Sb, and Hg
Smaller fly ash is inhalation risk, because less likely to be trapped in nose of larynx and more likely to be inhaled into deep recesses of lung
Ozone production in stratosphere
Concentration of O3 greatest at altitude 20 to 30 km
Formed when ordinary molecules O2 in the stratosphere absorb UV radiation from sun with wavelength of less than 240 m, causing them to dissociate into single oxygen atoms
Polyatomic molecules
molecules made up of more than two atoms or elements, held together by covalent bonds
All gases that contribute to greenhouse effect
Have numerous vibrations
Two most greenhouse gas polyatomic greenhouse gases CO2 and H2O
Approximately 89% of 34 K temperature increase resulting from greenhouse warming attributed to water. CO2 accounts for 7.5%
Radiative forcing
Decreases in stratospheric ozone concentration have cause cooling, while increases in tropospheric ozone concentration have caused warming
Aerosol particles affect radiative forcing:
Aerosols can reflect incoming solar radiation, which causes negative forcing
They can absorb IR radiation that would be lost from surface of Earth ,causing positive forcing
If radiative forcing for all aerosols is summed, effect of aerosols is negative
VOC/NOx ratio
At high ratio of VOC to NOx concentrations, OH will react mainly with VOCs,
At low ratio NOx reaction can predominate
air pollution sources
Transportation and industry responsible for 50% of air pollution from anthropogenic sources
Automobiles emit NOx, HCs, and CO
Burning of fossil fuels by stationary sources account for one third of air pollutants, in the form of sulfur oxides
Terpenes
volatile, unsaturated 5-carbon cyclic compounds
Nitrous oxide
Absorbs IR radiation in the 3 to 5 um and 7.5 to 9 um (atmospheric window) region
Concentration of nitrous oxide in atmosphere is 314 ppbv and increasing by 0.3% per year
Major source is released from soil, lakes, oceans, by microbial denitrification of nitrate
Positive feedback
Occurs when global warming increases evaporation from oceans that leads to higher concentrations of water vapor in air
Increased amount of water vapor causes more infrared radiation to be absorbed, and increases warming of Earth’s surface
Mass composition of atmosphere
Troposphere and stratosphere account for 99.9%
Half of mass concentrated within 6 km of Earth’s surface
Air above each square inch of surface at sea level exerts 14.7 pounds
Free troposphere
continuously radiates energy upward, cooling upper troposphere
Troposphere itself does not efficiently absorb solar radiation
Region receives warmed air that rises from surface
WHO guideline of particles
PM2.5 = 15 ug/m3 for 24 hour average concentration and 5 ug/m3 for annual mean
Solubility pump
Oceans remove CO2 from atmosphere by dissolving gas
Cold polar ocean water at surface dissolves atmospheric CO2
CO2 rich surface water sinks into deep ocean where it is sequestered for several hundred years
Sink is expected to become less important as polar regions become warmer
LIDAR
Light detection and ranging, similar to radar
Radar transmitter sends radio waves, and radar detector measures time it takes to bounce off an object and return to detector
Operates in the visible and infrared regions
Transmitter is laser, receiver is optical telescope that is focused on an extremely sensitive photomultiplier detector
Detector records photons that reach it and converts response to electronic signal
vegetation
Nonsoil component of biosphere
Absorbs CO2 during photosynthesis and stores carbon in biomass
30% of Earth’s surface is land and only 30% of land covered by forests
Vegetation is smaller sink than ocean and slightly smaller than soil
Within biosphere, most critical region for sequestration is tropical rain forest
Negative feedback
Occurs as troposphere becomes cloudier, and that causes more reflection of incident solar flux, causing cooling of Earth’s surface
Atmospheric Composition
Nitrogen 78% oxygen 21%
Argon 0.93% carbon dioxide 0.037%
Smaller amounts of neon, helium, krypton, methane
Catalytic converter basics
At 25C, efficiency near zero
Ineffective when starting a cold engine, and low during warm up
Lead-free gasoline must be used because lead coats and inactivates catalysts
Today’s catalytic converters remove 96% of CO and HCs and 76% of NOx from exhausts
Exposure and treatment for CO poisoning
Inhalation of pure oxygen, reversing direction of reaction
Symptoms of CO poisoning: headache, dizziness, impaired judgement, drowsiness, slowed reflexes, respiratory failure, loss of consciousness and death
Prolong explore to CO levels as low as 10 ppm can be harmful
Colourless, tasteless, and odourless
Concentration may reach 50 ppm on streets, and higher underground garages/traffic
Absorbance
Directly proportional to concentration C of the absorbing molecule in sample
aerosols
Minute particles with diameters of less than approximately 10um
Have very large surface areas that act as sites for chemical interactions
Chemical reactions may occur at surface or within
particulates
a mixture of particles in the air, larger than aerosols
greenhouse effect
Warming effect caused by absorption and reradiating of IR radiation by greenhouse gases
Gases act like glass in a greenhouse
Visible light passes through glass and is absorbed by objects inside greenhouse
Objects warmed and emit heat energy in form of IR radiation which cannot pass through glass, therefore heat is trapped inside
greenhouse gases: co2, water vapor, methane, nitrous oxide, cfcs, ozone
secondary organic aerosol formation
Principal atmospheric oxidants are hydorxyl radical (*OH) ozone (O3), and nitrate radical (NO3-)
In SOA formation, several generations of gas-phase oxidation occur, each generation producing compounds of decreasing volatility
Effects of CO on human health
Interferes with oxygen carrying capacity of blood.
Normally, Hemoglobin in red blood cells combine with oxygen to form OxyHemoglobin (HbO2)
HbO2 carried in bloodstream to parts of the body, where oxygen is released to tissues
CO binds stronger to Hv than oxygen. If CO is present, it displaces oxygen from Hb and reduces amount of oxygen that can be delivered to tissues
hydroxyl radicals
Continually formed and consumed in troposphere and are produced as a result of a series of complex reactions primarily involving ozone, water, and nitrogen dioxide
Play a role in removal of CO and HCs from atmosphere and in the formation of nitric acid, sulfuric acid, and photochemical smog from atmospheric gases
Aerosol particles
Effect of particulate matter on heat flux depends on particle size and not as much total concentration
Large dark particles absorb light and add to warming of atmosphere
Small particles scatter incident sunlight and increase the albedo of the atmosphere
Natural sources of light-scattering aerosols are estimated to produce 50% to 75% of all atmospheric aerosols
UV spectrometer for ozone measurement
Uses a low-pressure mercury lamp to produce electromagnetic radiation
Output of mercury lamp has a maximum at 254 nm, very near to absorbance maxima for ozone
Tube carrying air split into two streams
One passes through ozone scrubber removing ozone and air acts as reference
Other with ozone travels to sample cell
Each stream flows through one 50-cm sample cell
Light from mercury lamp sent down, silicon photodiode at end detects intensity
Ozone contraction 1ppm produces absorption 0.2
Troposphere
10-16 km above earth
Temperature decreases steadily as distance from Earth’s surface increases until -57C
Peroxyacetyl nitrate (PAN)
Component of smog that causes major irritation
Stable molecules with long lifetimes in cooler air, reservoir for NOx
PANs break down in warmer climates to release NO2 and releases ozone and hydroxyl radicals
Major natural source of aerosols
Ammonium sulphates generated during microbial degradation of decaying biomass and organic matter in soil and water
Reactive organic molecules that are released from natural sources
EX. release of organic molecules, terpenes, from coniferous trees.
Industrial sources of emissions
Waste incineration and kiln drying of cement emit metals
PM from industrial process contains Fe2O3, Fe3O4, Al2O3, SiO2, carbonates of several metals
Heavy metals vaporize at high temperature and then recondense onto particles that are formed simultaneously
Iron released into atmosphere far more than any other metal
Lead is emitted form lead-ore smelters and lead-acid battery manufacturing
Fate of atmospheric NOx
NO2, regardless of source, is ultimately removed from atmosphere as nitric acid and nitrate in dust and rainfall
NO2 combines with water vapor to form nitric acid
Much of nitric acid in atmosphere formed within aqueous aerosols
Some nitric acid form reacts with ammonia and metallic particles to form ammonium nitrate
Nitrates dissolve in rain and snow or settle as particles, contributing to acid deposition
secondary air pollutants
Harmful substances produced by chemical reactions between primary pollutants and other constituents in atmosphere
e.g. Sulfuric acid, nitric acid, sulfates, and nitrates, and ozone, and other photochemical oxidants
POA
Primary organic aerosols, emitted directly as particles from combustion sources such as transportation and biomass burning remaining in particulate form, serving as inert nuclei in which secondary organic components condense
Sufficiently volatile that it evaporates after emission
Once emitted, is diluted and evaporates, then resulting semivolatile organic vapors react in gas phase with OH radical and other atmospheric oxidants, forming low-volatility oxidation products
Evaporation-reaction-recondensation cycle leads to significant changes in chemical nature of primary OA and is one of the reasons why organic aerosol in large urban areas with considerable organic particulate emissions is dominated by oxygen containing compounds
Blue light
Shorter wavelength, more scattered than red
Sky, seen in scattered light = blue
Sunset, seen in transmitted light = red
Average solar flux reacting the earth (at the top of the stratosphere)
1368 W/m2
Average surface temperature of the Earth
288 K (15C)
Temperature above mesosphere
Rises to 1200C in atmosphere
Caused by few gaseous molecules in thermosphere absorbing most energetic radiation emanating from sun
biological pump
Marine phytoplankton take in atmospheric CO2 and released carbon in a form that sinks to ocean floor where it is sequestered through lithification of sedimentary rocks
Process estimated to reduce atmospheric CO2 by ⅓
Electrostatic precipitation
Method to control particulate emissions
Gases and particulate matter passed through high-voltage chamber before leaving chimney stacks
Negatively charger central electrode imparts a negative charge to particles
Then attracted to positively charged walls of chamber
As charges neutralized, particle clump together and fall to bottom to collect
TSP test
Total suspended particle test measuring total amount of suspended particle matter
Air drawn through pre weighed filter at rate of 1 m3h-1
Total time air pumped is recorded so total volume of air passing known
At end, filter weight again so total weight of particulate matter known
Montreal protocol
1987 first international effort to protect ozone layer, calling for CFC production to be cut back to 5%
Amended in 1992, when 140 nations agreed to end CFC production by 1995 and speed phaseout of ozone-depleting chemicals
Target chemicals: CCl4, CH3Br, HCFCs
CFC-11 and CFC-12 lifetimes approximately 55 and 116 years