AP Chemistry VSEPR Model Names
1/14
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
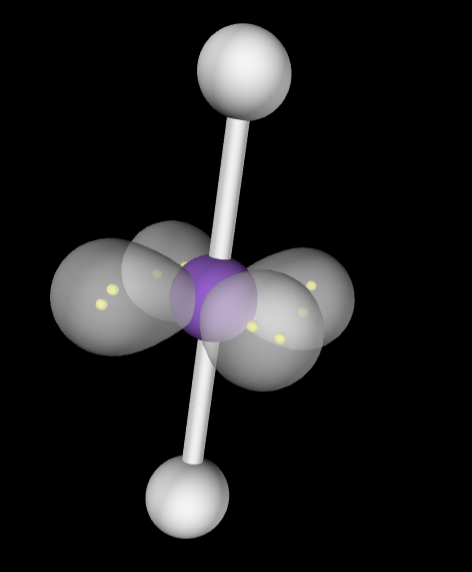
Electron Geometry: Linear
Molecular Geometry: Linear, 180 degrees
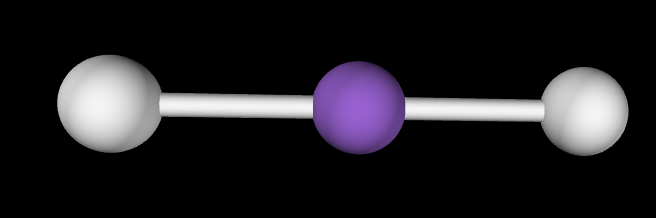
Electron Geometry: Trigonal Planar, 0 lone pairs
Molecular Geometry: Trigonal Planar, 120 degrees
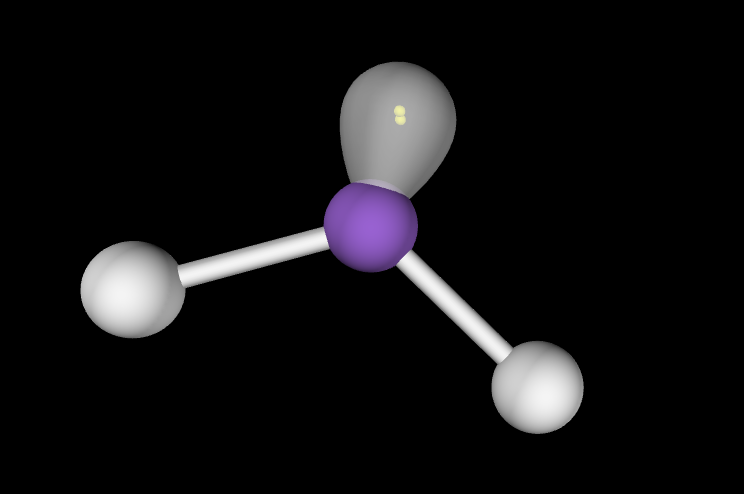
Electron Geometry: Trigonal Planar, 1 lone pair
Molecular Geometry: Bent, slightly less than 120 degrees
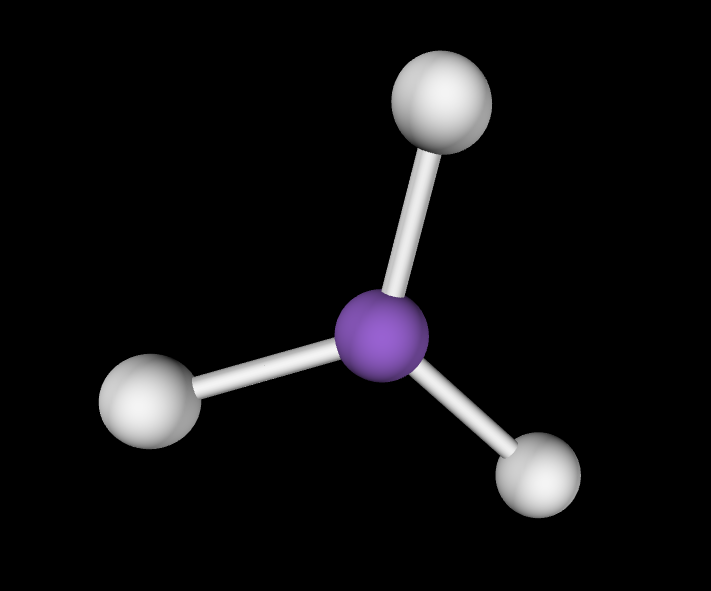
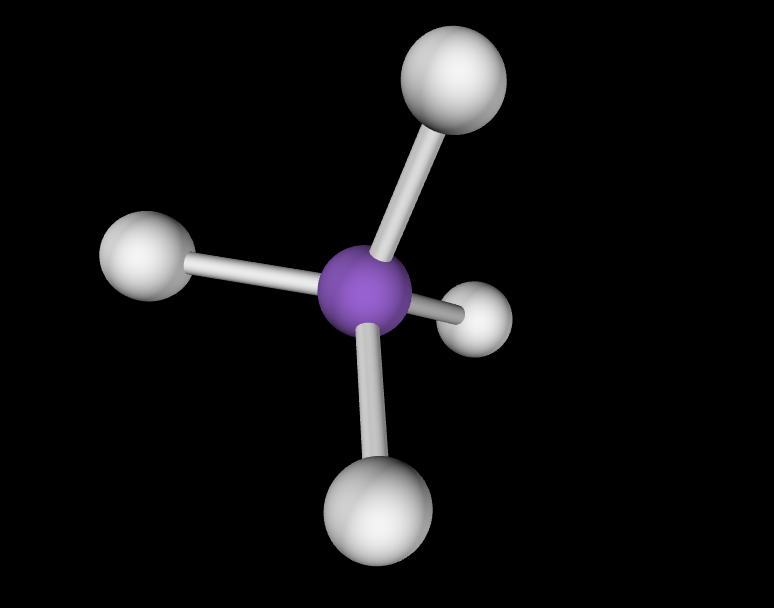
Electron Geometry: Tetrahedral, 0 lone pairs
Molecular Geometry: Tetrahedral, 109.5 degrees
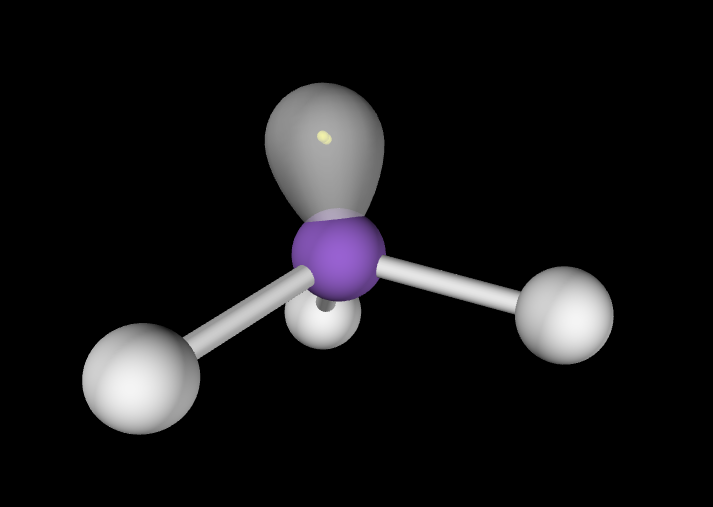
Electron Geometry: Tetrahedral, 1 lone pair
Molecular Geometry: Trigonal Pyramidal, slightly less than 109.5 degrees
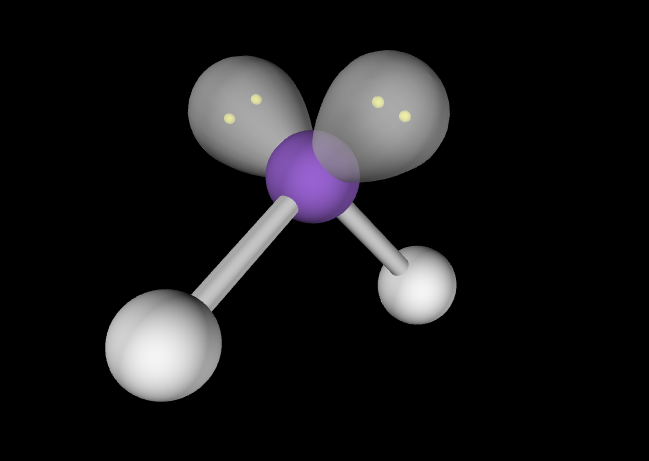
Electron Geometry: Tetrahedral, 2 lone pairs
Molecular Geometry: Bent, slightly less than 109.5 degrees
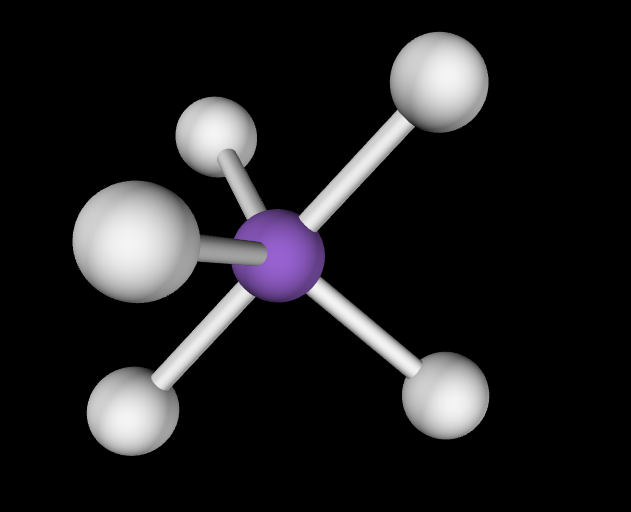
Electron Geometry: Trigonal Bipyramidal, 0 lone pairs
Molecular Geometry: Trigonal Bipyramidal, angles of 120 degrees and 90 degrees
Electron Geometry: Trigonal Bipyramidal, 1 lone pair
Molecular Geometry: See-saw, lone pair in the set of trigonal planar atoms
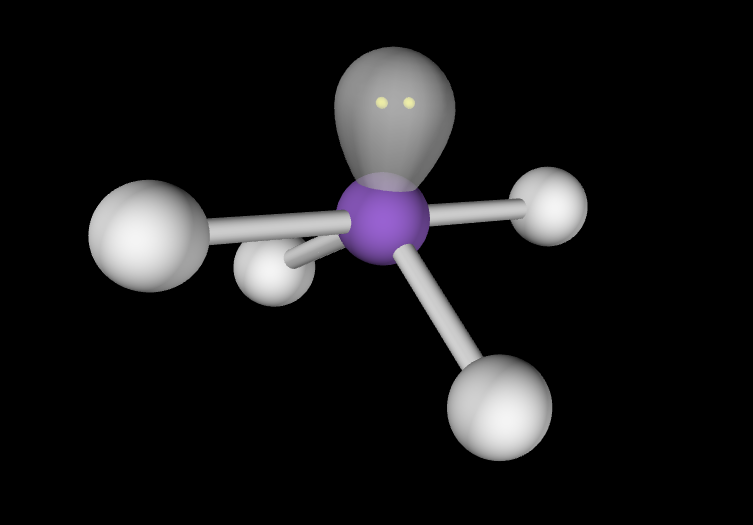
Electron Geometry: Trigonal Bipyramidal, 2 lone pairs
Molecular Geometry: T-Shaped
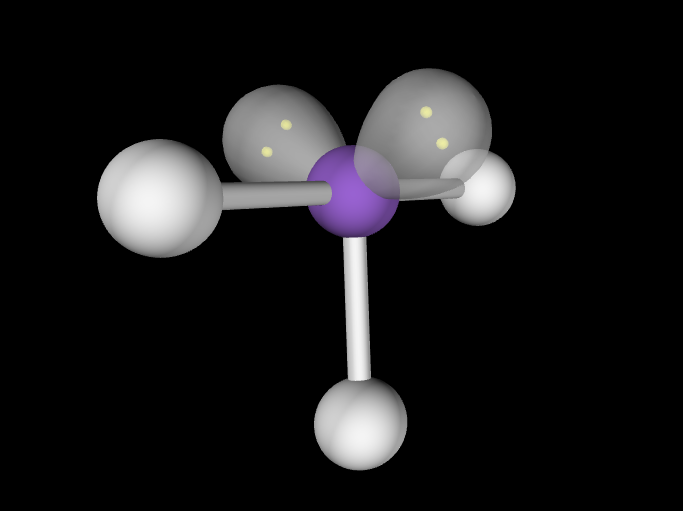
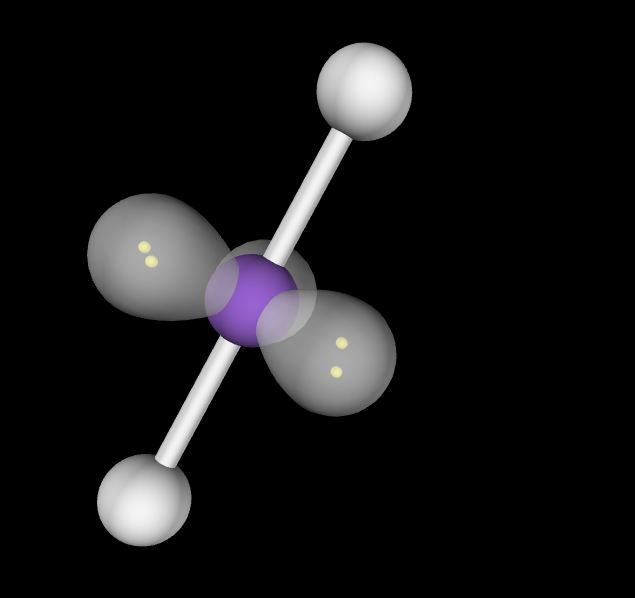
Electron Geometry: Trigonal Bipyramidal, 3 lone pairs
Molecular Geometry: Linear
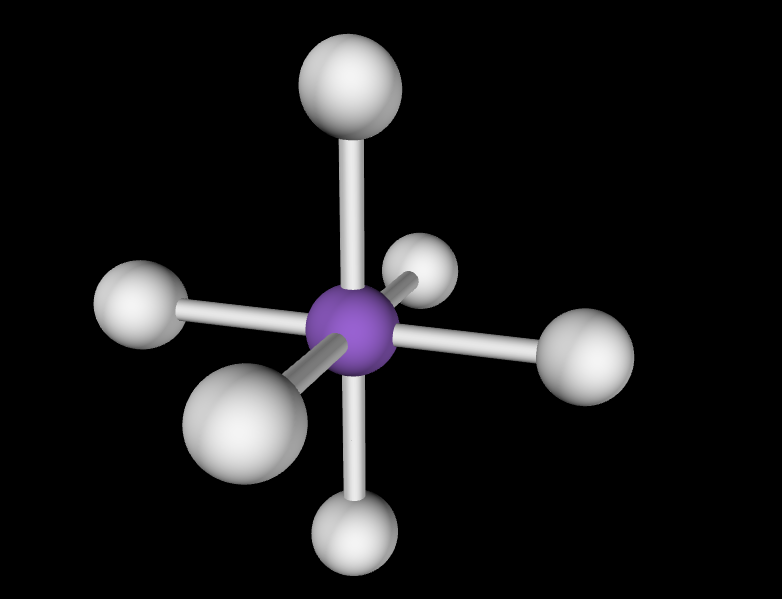
Electron Geometry: Octahedral, 0 lone pairs
Molecular Geometry: Octahedral, 90 degree angles
Electron Geometry: Octahedral, 1 lone pair
Molecular Geometry: Square Pyramidal
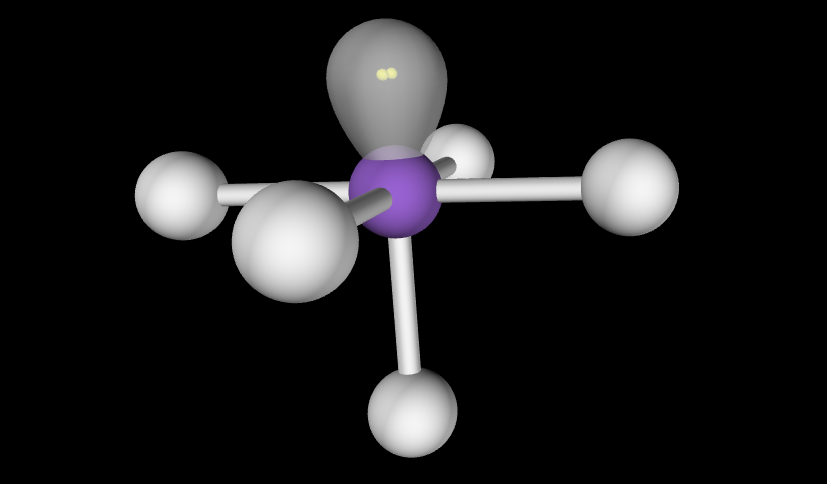
Electron Geometry: Octahedral, 2 lone pairs
Molecular Geometry: Square Planar, lone pairs above and below square plane
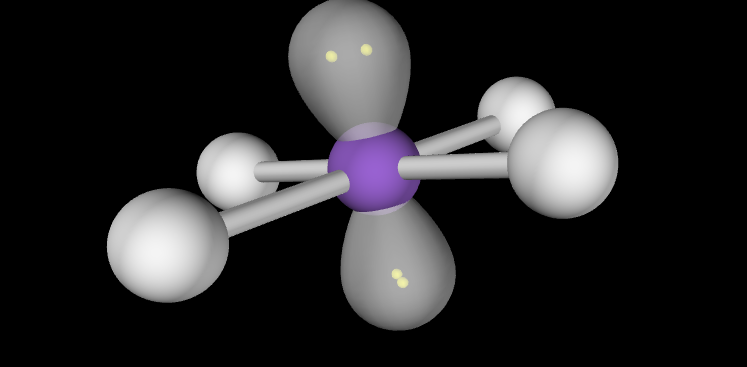
Electron Geometry: Octahedral, 3 lone pairs
Molecular Geometry: T-Shaped
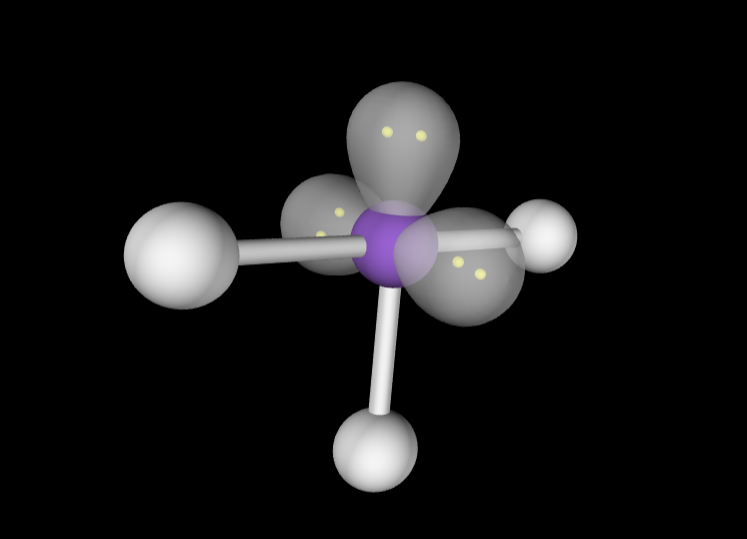
Electron Geometry: Octahedral, 4 lone pairs
Molecular Geometry: Linear